- Title
-
Pannexin 1 drives efficient epithelial repair after tissue injury
- Authors
- Lucas, C.D., Medina, C.B., Bruton, F.A., Dorward, D.A., Raymond, M.H., Tufan, T., Etchegaray, J.I., Barron, B., Oremek, M.E.M., Arandjelovic, S., Farber, E., Onngut-Gumuscu, S., Ke, E., Whyte, M.K.B., Rossi, A.G., Ravichandran, K.S.
- Source
- Full text @ Sci Immunol
|
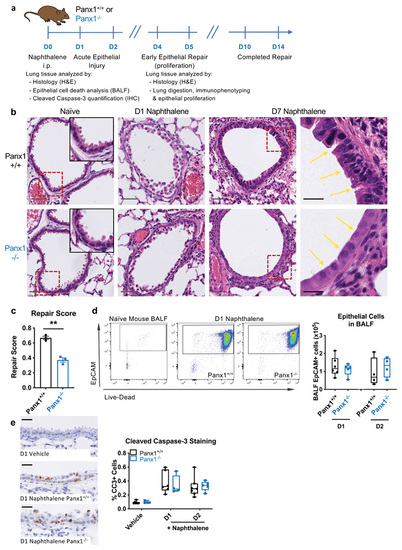
(a) Wild type (Panx1+/+) or pannexin 1 globally deficient mice (Panx1-/-) were injected with naphthalene (200mg/kg) i.p. to cause acute airway epithelial death prior to analysis of bronchoalveolar lavage fluid (BALF) or lung tissue. (b) Representative H&E staining of lung tissue sections from naïve Panx1+/+ or Panx1-/- mice and at day 1 (D1) and D7 post-naphthalene injury, with injured and repairing airway epithelium highlighted by yellow arrows. Grey scale bars, 50μm; black scale bars, 20μm. Dotted red lines reflect insets in naïve images and zoomed in images in D7 naphthalene images. (c) H&E sections were blinded prior to analysis by a lung pathologist (n=3 per group, assessed by t-test, one independent experiment). (d) BALF was acquired at day 1 or day 2 post-naphthalene injury and cellular content analysed for epithelial cells (CD45-/EpCAM+ cells) and their staining with a live-dead (LD) marker (low staining of LD marker are live cells, high staining are dead cells; n=4-6, two independent experiments). (e) Caspase-3 activation was assessed by cleaved caspase-3 staining on lung tissue sections in the absence of injury and on days 1 and 2 after naphthalene epithelial injury (n=4-7, two independent experiments). Black scale bars, 20um. **p<0.01. |
|
(a-c) |
|
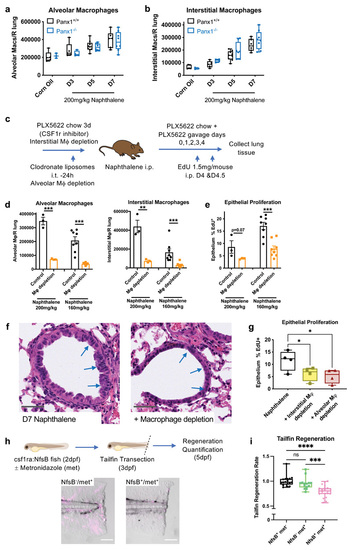
(a) Lung alveolar macrophage numbers (CD45+/CD11c+/Siglec-F+ cells) and (b) interstitial macrophage numbers (CD45+/CD11b+/CD64+ cells) in digested right lung lobes after naphthalene-induced epithelial injury (200mg/kg) analysed by flow cytometry of lung digests in Panx1+/+ and Panx1-/- mice (n=3-5 corn oil; n=5-9 post-naphthalene). (c) Schema of experimental protocol for lung macrophage depletion in Panx1+/+ mice, achieved by intratracheal (i.t.) administration of 100μL clodronate liposomes to deplete alveolar macrophages, and PLX5622 containing chow (1200mg/kg feed) ad libitum for 3d prior to experimentation with additional PLX5622 (65mg/kg) given by oral gavage (on days 0,1,2,3&4 of naphthalene administration) to deplete interstitial macrophages. (d) Confirmation of macrophage depletion after epithelial injury and (e) |
|
(a) Schema of experimental protocol for deleting Panx1 using CX3Cr1-Cre or LysM-Cre mediated approaches prior to epithelial injury, with (b) |
|
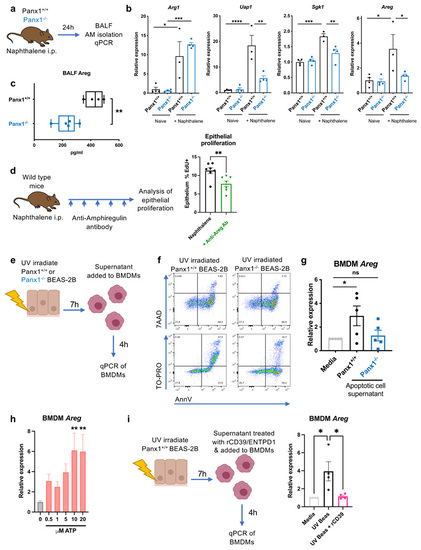
(a) Schema of experimental protocol for analysis of gene expression in lung alveolar macrophages at day 1 after naphthalene-induced epithelial injury in Panx1+/+ and Panx1-/- mice. (b) qPCR analysis of |
|
(a) Schema of experimental protocol for RNAseq analysis of purified lung epithelium from Panx1+/+ and Panx1-/- mice after naphthalene epithelial injury with (b) heat map of differentially regulated genes, units representing log2 scaled counts. (c) CRISPR–Cas9 genetic deletion of |