- Title
-
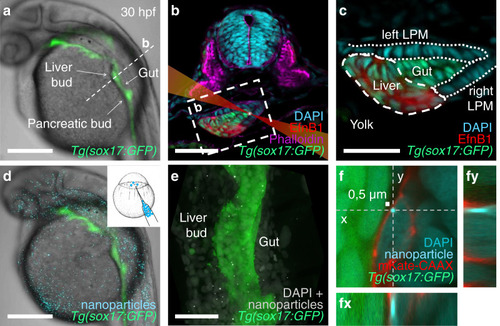
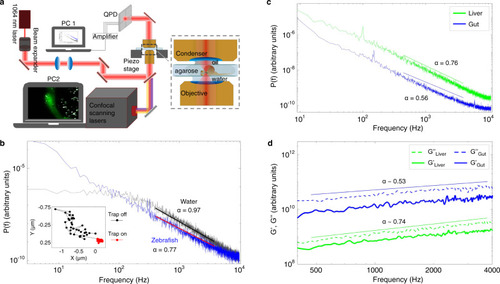
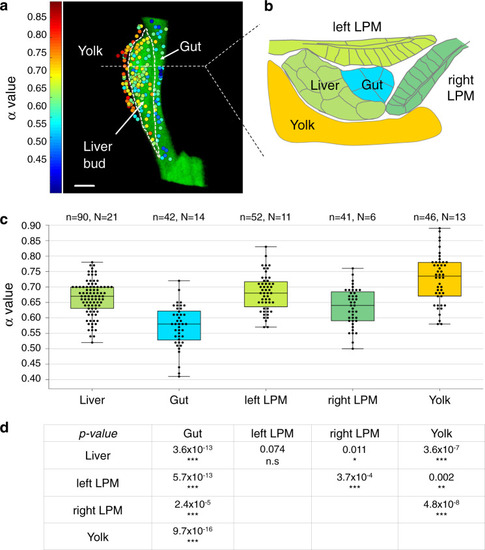
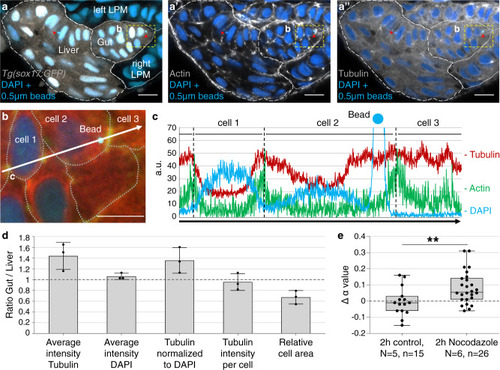
Foregut organ progenitors and their niche display distinct viscoelastic properties in vivo during early morphogenesis stages
- Authors
- Dzementsei, A., Barooji, Y.F., Ober, E.A., Oddershede, L.B.
- Source
- Full text @ Commun Biol
|
|
|
|
|
|
|
|