- Title
-
Differential Requirement of Gata2a and Gata2b for Primitive and Definitive Myeloid Development in Zebrafish
- Authors
- Peña, O.A., Lubin, A., Rowell, J., Hoade, Y., Khokhar, N., Lemmik, H., Mahony, C., Dace, P., Umamahesan, C., Payne, E.M.
- Source
- Full text @ Front Cell Dev Biol

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
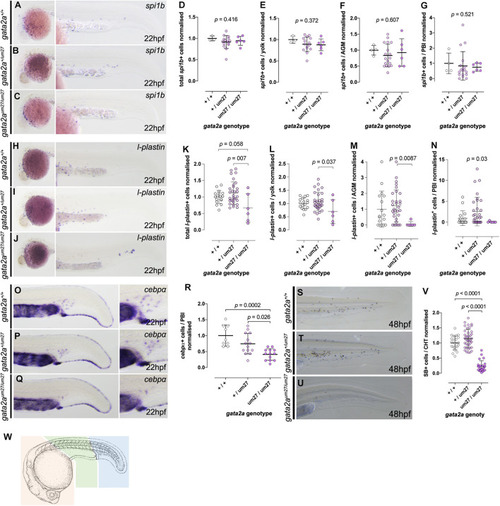
Primitive myeloid development in gata2a mutants at 22 hpf. (A–C) Expression of spi1b by in situ hybridization in 22 hpf embryos. (A–C) Lateral views of the head of the embryos (left) and lateral views of the tails (right), showing normal numbers of myeloid cells. (D–G) Quantifications of total (D) spi1b+ cells, and in the yolk (E), AGM (F), and the PBI (G). (H–J) Expression of l-plastin by in situ hybridization in 22 hpf embryos. (H–J) Lateral views of the head of the embryos (left) and lateral views of the tails (right) show decreased l-plastin+cells in gata2aum27/um27 homozygotes. (K–N) Quantifications of l-plastin+cells in the whole embryo (K), in the yolk (L), AGM (M), and the PBI (N). (O–Q) Lateral views showing cebpa expression in the tail (left) and PBI (right) in 22 hpf embryos. (R) Quantification of cebpa+ cells in the PBI of Gata2aum27 mutants. (S–U) Lateral views of 48 hpf mutant embryos stained with SB, which labels granulocytes. (V) Quantification of SB+ cells in the CHT of 48 hpf embryos shows decreased number of granulocytes in gata2aum27/um27 homozygotes. (W) Cartoon scheme of location of cells counted in these studies. Orange region = yolk, Green = AGM, blue = PBI. Camera lucida image modified from Kimmel et al. (1995). EXPRESSION / LABELING:
PHENOTYPE:
|
|
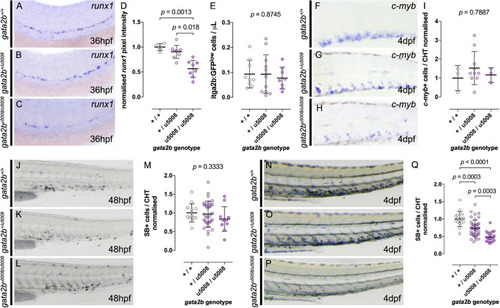
Impaired HSPC development in gata2a mutants. (A–C) Expression of runx1 by in situ hybridization in 36 hpf embryos, showing decreased expression in gata2aum27/um27 homozygotes. (D–F) Lateral views of c-myb WISH on 36 hpf Gata2aum27 mutants showing decreased expression in in gata2aum27/um27 homozygotes. (G) Quantification of c-myb expression from in situ hybridization images in the AGM of 36 hpf embryos. In (H) mutant fish carrying itga2b:GFP transgene, labeling HSPCs that reside in the CHT, were used to quantify GFPlow cells in the CHT of 56 hpf embryos, showing an allele dependent decrease in GFPlow cells. (I–K) Lateral views of 4 dpf larvae showing the expression of c-myb by in situ hybridization. (L) Quantification of c-myb+ cells in the CHT of 4 dpf mutant larvae. (M–O) Lateral views of the tails of 4 dpf mutant Tg(itga2b:GFP) fish showing a decrease of GFP+ cells in gata2aum27/um27 homozygotes. In (P), the presence (up) or absence (bottom) of blood flow in the tail of Gata2aum27 mutant fish was monitored each day until 5 dpf. Notice the gata2aum27/um27 homozygotes (purple triangles) gaining blood circulation at 3 and 5 dpf. (Q) Plot showing the correlation of the presence (up) or absence (bottom) of blood flow in the tail of Gata2aum27 mutant fish and the presence (right) or absence (left) of Tg(itga2b:GFP)+ cells in 4 dpf fish. At 4 dpf, some gata2aum27/um27 homozygotes (purple triangles) show GFP+ cells, all of which also exhibit circulation in the tail. (R–R″) Lateral views of 4 dpf mutant Tg(itga2b:GFP) fish showing a range of phenotypes of gata2aum27/um27 homozygotes. (S–U) Lateral views of 4 dpf mutant larvae stained with SB. (V) Quantification of SB+ cells in the CHT of 4 dpf larvae shows decreased number of granulocytes in gata2aum27/um27 homozygotes. |
|
Gata2b is necessary for HSPC specification but dispensable for HSPC maturation. (A–C) Analysis of runx1 expression at 36 hpf by in situ hybridization in Gata2bu5008 mutant embryos shows decreased expression in gata2bu5008/u5008 homozygotes. (D) Quantification of runx1 expression in the AGM of 36 hpf mutant embryos shows allele dependent decrease of runx1 expression. (E) Number of itga2b:GFPlo cells by flow cytometry of individual Tg(itga2b:GFP) Gata2bu5008 mutant embryos at 3 dpf. (F–H) Expression of c-myb by in situ hybridization in the CHT of 4 dpf mutant larvae. (I) Quantification of c-myb+ cells in the CHT of 4 dpf mutant fish. In (J–L), lateral views of 48 hpf mutant embryos stained with SB, labeling granulocytes. (M) Quantification of SB+ cells in the CHT of 48 hpf mutant embryos. In (N–P), SB stainings of 4 dpf larvae show allele dependent decrease of SB+ cells in the CHT of Gata2bu5008 mutant fish, quantified in (Q). |
|
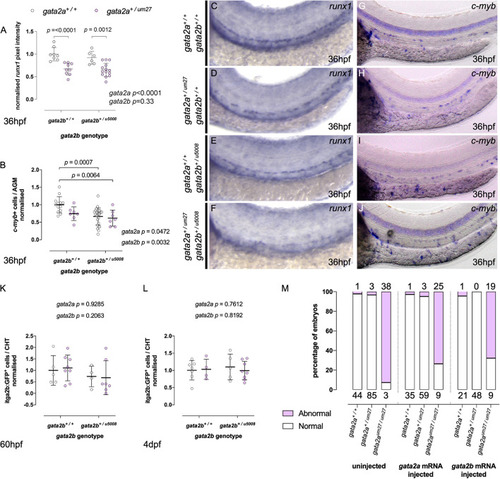
Gata2aum27 and Gata2bu5008 mutations have independent and non-additive effects on HSPC specification. (A,B) Quantifications of runx1 expression (A) and c-myb+ cells (B) in the AGM of 36 hpf of embryos from Gata2a+/um27 to Gata2b+/u5008 crosses. (C–J) Expression of runx1 (C–F) and c-myb (G–J) by in situ hybridization in 36 hpf embryos. In (K,L), mutant fish carrying itga2b:GFP transgene, labeling HSPCs in the CHT, were used to quantify stationary GFPlo cells in the CHT of 60 hpf (K) and 4 dpf (L) fish. (M) The percentage of embryos displaying blood flow in the trunk in embryos injected with Gata2a (middle), Gata2b (right) mRNA is compared to that of uninjected embryos (left). Absolute numbers of abnormal (pink) and normal (white) embryos are indicated above and under the columns, respectively. EXPRESSION / LABELING:
PHENOTYPE:
|
|
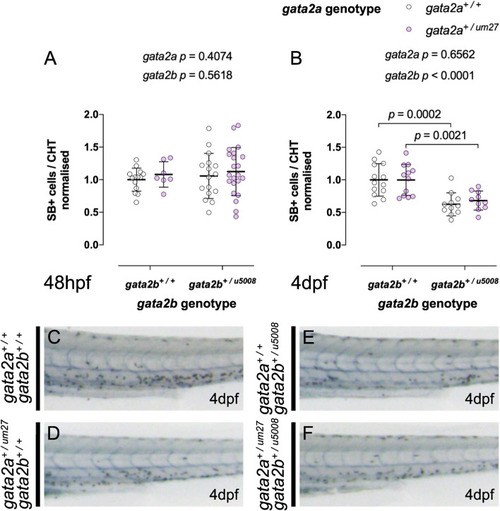
Gata2b+/u5008 mutants display impaired definitive granulopoiesis independent of gata2a genotype. (A,B) Quantifications of SB+ cells in the CHT of 48 hpf (A) and 4 dpf (B) fish from a Gata2a+/um27 to Gata2b+/u5008 cross. (C–F) Lateral views of the trunk of SB stained larvae at 4 dpf showing decreased SB+ cells in the CHT of Gata2b+/u5008 fish. |