- Title
-
The Zebrafish Meiotic Cohesin Complex Protein Smc1b Is Required for Key Events in Meiotic Prophase I
- Authors
- Islam, K.N., Modi, M.M., Siegfried, K.R.
- Source
- Full text @ Front Cell Dev Biol
|
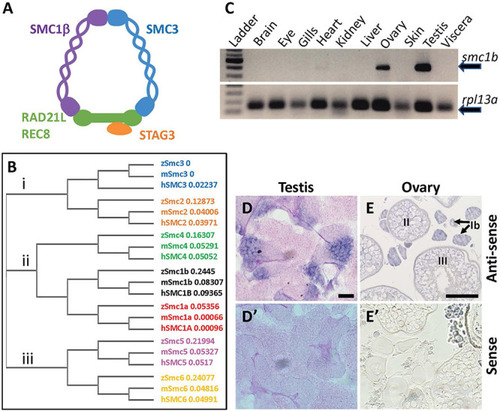
Zebrafish EXPRESSION / LABELING:
|
|
|
|
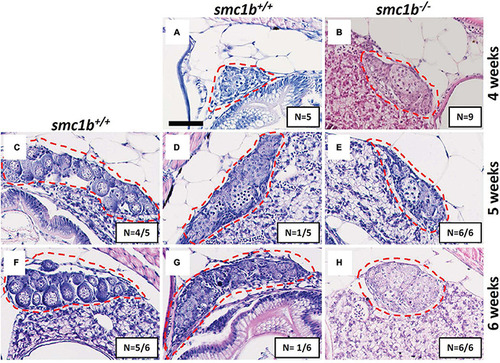
Absence of oogenesis in PHENOTYPE:
|
|
Spermatocytes in |
|
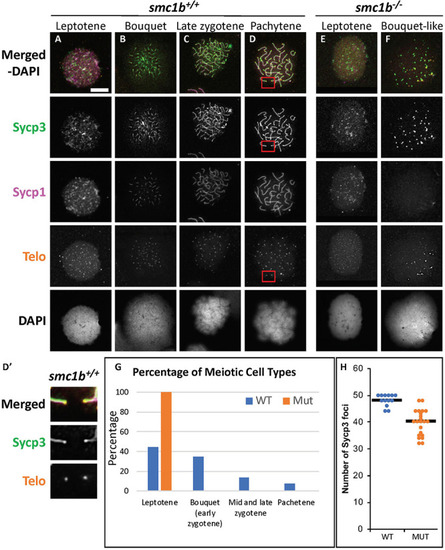
Failed synapsis and anomalous pairing in |
|
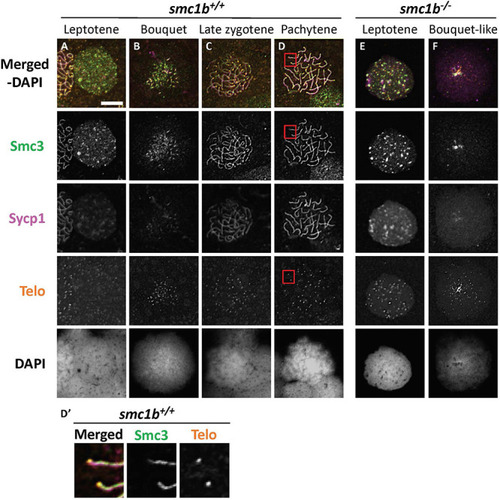
The cohesin complex does not associate with meiotic chromosomes in zebrafish spermatocytes. |
|
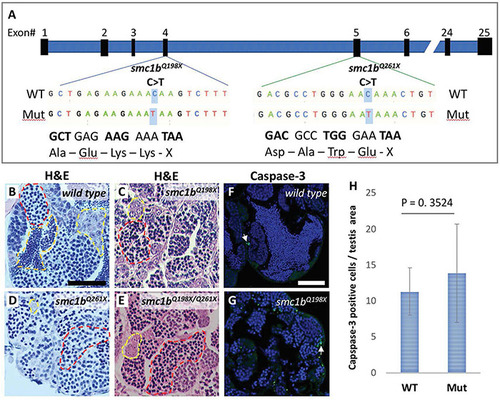
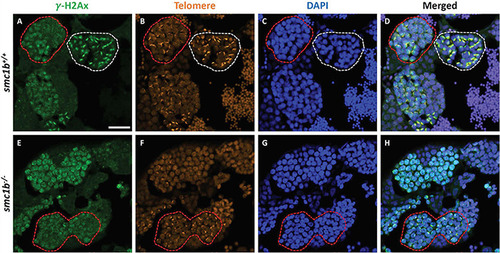
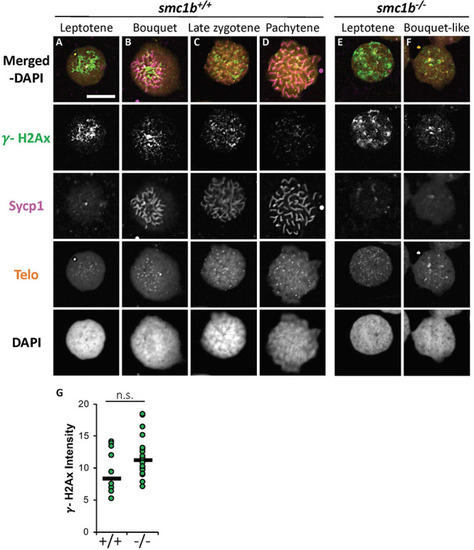
DNA double strand break marker γ–H2Ax was expressed in PHENOTYPE:
|
|
DNA double strand break marker γ-H2Ax was expressed in |
|
DNA double strand break and recombination marker Rad51 was expressed in |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|