- Title
-
Smoothened transduces Hedgehog signals via activity-dependent sequestration of PKA catalytic subunits
- Authors
- Arveseth, C.D., Happ, J.T., Hedeen, D.S., Zhu, J.F., Capener, J.L., Klatt Shaw, D., Deshpande, I., Liang, J., Xu, J., Stubben, S.L., Nelson, I.B., Walker, M.F., Kawakami, K., Inoue, A., Krogan, N.J., Grunwald, D.J., Hüttenhain, R., Manglik, A., Myers, B.R.
- Source
- Full text @ PLoS Biol.
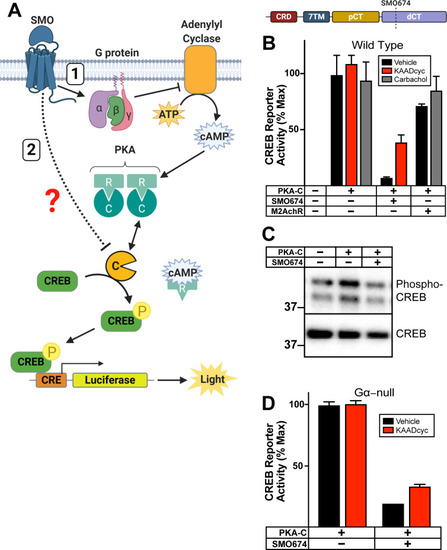
|
( |
|
HEK293 cells expressing ( |
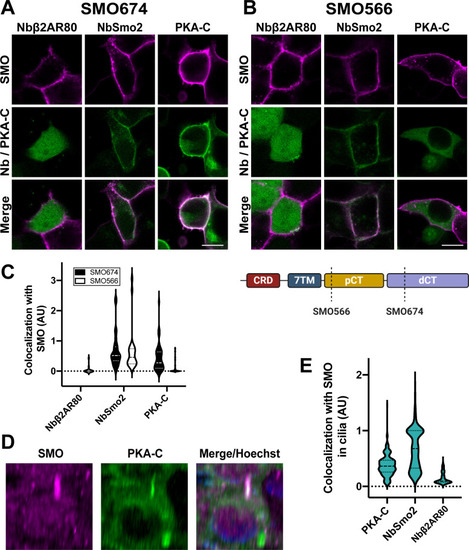
|
( |
|
( |
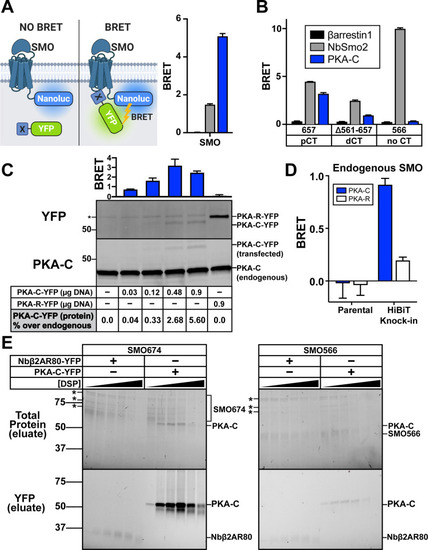
|
( |
|
( |
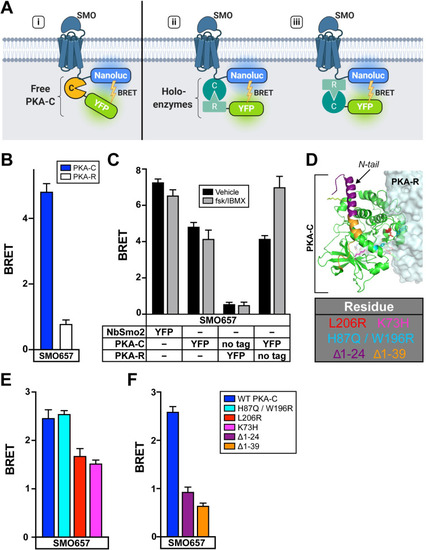
|
( |