- Title
-
Differential Regenerative Capacity of the Optic Tectum of Adult Medaka and Zebrafish
- Authors
- Shimizu, Y., Kawasaki, T.
- Source
- Full text @ Front Cell Dev Biol
|
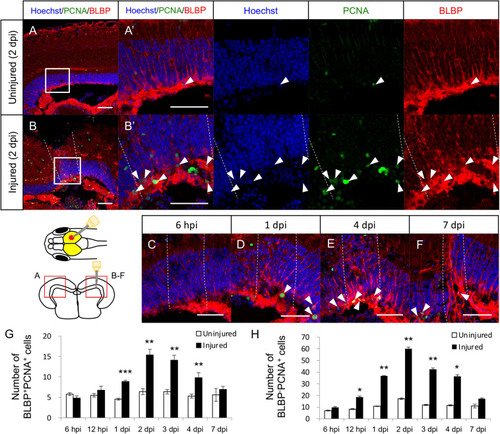
Proliferation of radial glia (RG) is increased in response to stab wound injury. Representative images of proliferative RG (BLBP+PCNA+ cells) in the uninjured |
|
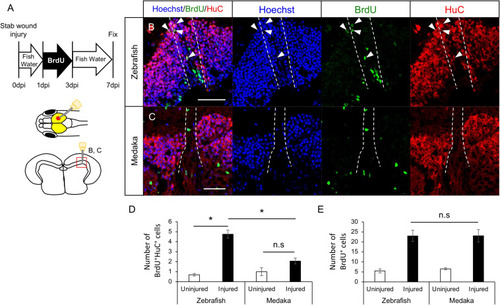
Generation of newborn neurons in the injured medaka is limited compared with zebrafish. |
|
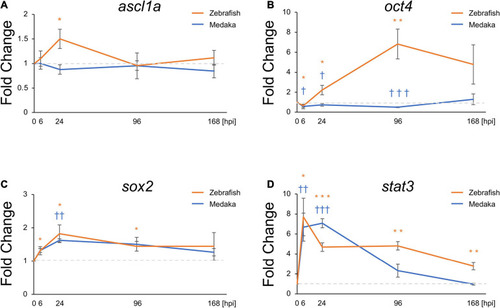
Pro-regenerative transcriptional factors are differentially expressed between the injured medaka and zebrafish. Quantitative polymerase chain reaction analysis of the pro-regenerative transcriptional factors ascl1a |
|
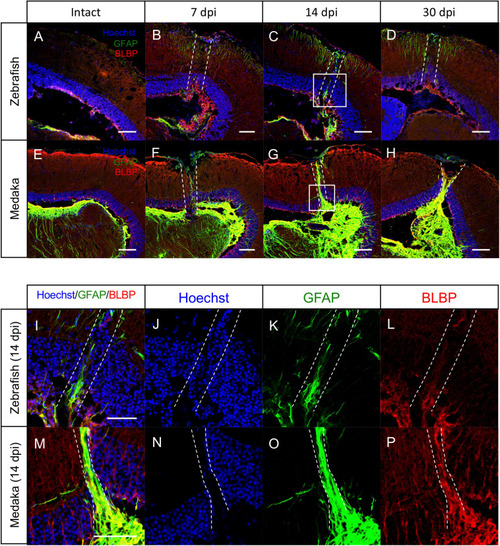
Persistent glial scar-like structure is observed in the injured medaka tectum. |