- Title
-
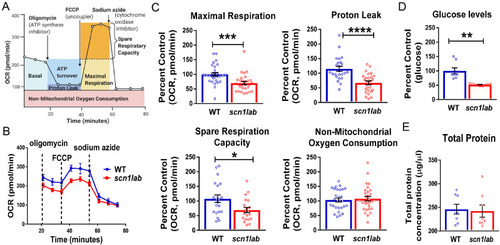
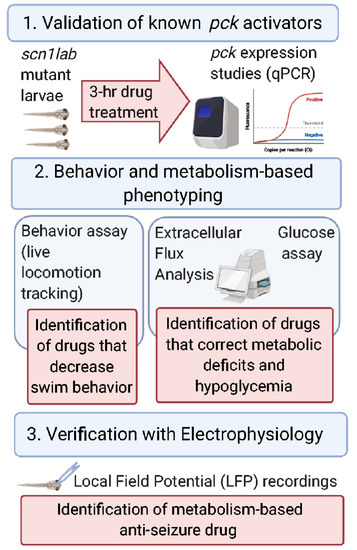
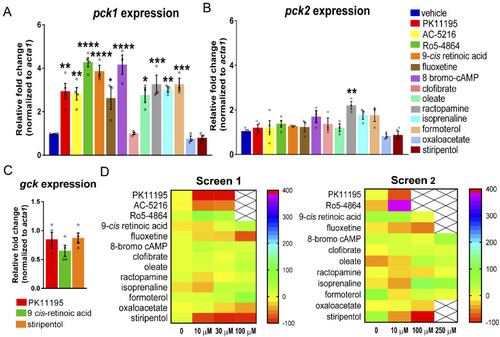
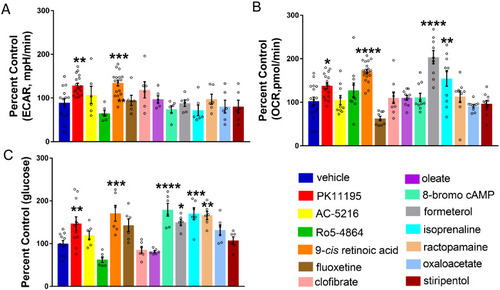
Enhancing glucose metabolism via gluconeogenesis is therapeutic in a zebrafish model of Dravet syndrome
- Authors
- Banerji, R., Huynh, C., Figueroa, F., Dinday, M.T., Baraban, S.C., Patel, M.
- Source
- Full text @ Brain Commun
|
|
|
|
|
|
|
|
|
|
|
|