- Title
-
Subcellular Dissection of a Simple Neural Circuit: Functional Domains of the Mauthner-Cell During Habituation
- Authors
- Bátora, D., Zsigmond, Á., Lőrincz, I.Z., Szegvári, G., Varga, M., Málnási-Csizmadia, A.
- Source
- Full text @ Front. Neural Circuits
|
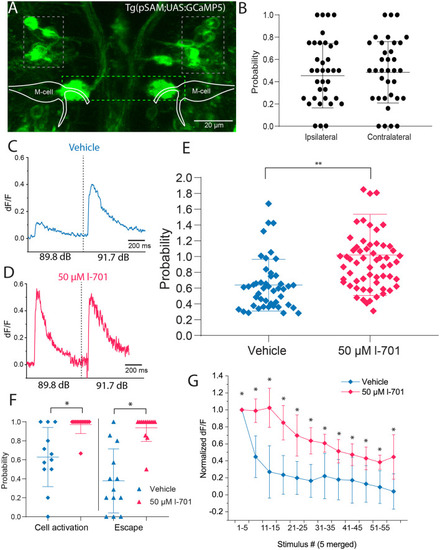
Characteristics of the M-cell mediated escape response, and its habituation. |
|
|
|
Differential properties of the LD and the AIS during setting the startle threshold. |
|
Dynamics of glutamate release in response to repetitive acoustic stimulation exhibit distinct properties at the LD and the AIS. Dynamics of presynaptic glutamate-release in response to repetitive acoustic stimulation at the regions of interest. The fish received 60 acoustic stimuli (91.7 dB) at frequencies ranging from 1 Hz to 4 Hz. Fluorescence values were recorded from both regions, peak fluorescence values of every five consecutive glutamate spike events were averaged and normalized to the mean of peak fluorescence values of the first five stimuli. Increasing the frequency of stimulation revealed different underlying mechanisms of depression in the two regions. |
|
Spiral fiber neuron activity is modulated by NMDA receptor blockade in setting the startle threshold and habituation. |
|
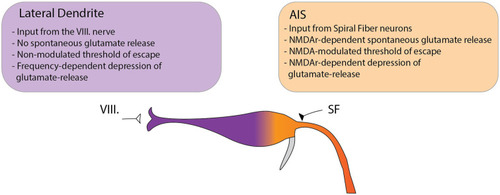
Model depicting the differential functions of glutamatergic Mauthner-cell inputs in setting the startle threshold and habituation. The M-cell receives two major glutamatergic inputs: the VIII nerve ending at the distal LD and input from SFNs ending at the proximal AIS. We observed several key differences between the two sites suggesting distinct roles in modulating the startle behavior. At the LD no spontaneous subthreshold glutamate release was observed. In setting the threshold to the startle reflex in response to acoustic stimulation, the release at the LD could not be modulated by NMDA receptor antagonism. Furthermore, the rate of depression of glutamate release to repeated stimulation was strongly dependent on the frequency of the stimuli but was not altered by NMDA receptor antagonism. In contrast, at the AIS spontaneous release events were frequently observed and could be increased by NMDA receptor blockade. In setting the startle threshold, glutamate release at the AIS strongly correlated with the probability of escape and was dependent on NMDA receptor activity. Additionally, NMDA receptor blockade strongly modulated the depression of glutamate release in response to repetitive acoustic stimulation. |