- Title
-
Synergistic roles of Wnt modulators R-spondin2 and R-spondin3 in craniofacial morphogenesis and dental development
- Authors
- Alhazmi, N., Carroll, S.H., Kawasaki, K., Woronowicz, K.C., Hallett, S.A., Macias Trevino, C., Li, E.B., Baron, R., Gori, F., Yelick, P.C., Harris, M.P., Liao, E.C.
- Source
- Full text @ Sci. Rep.
|
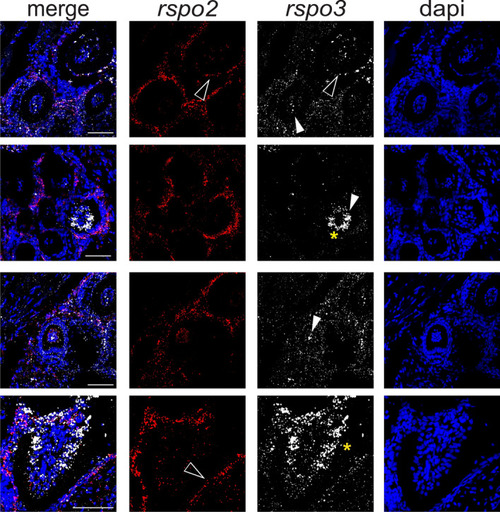
High resolution gene expression analysis detected dynamic spatiotemporal localization of EXPRESSION / LABELING:
|
|
RNAscope gene expression analysis of |
|
|
|
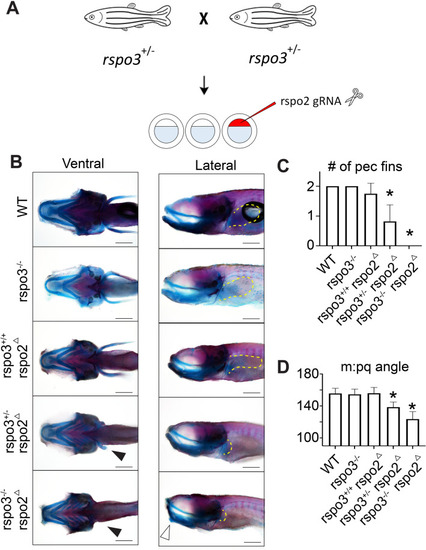
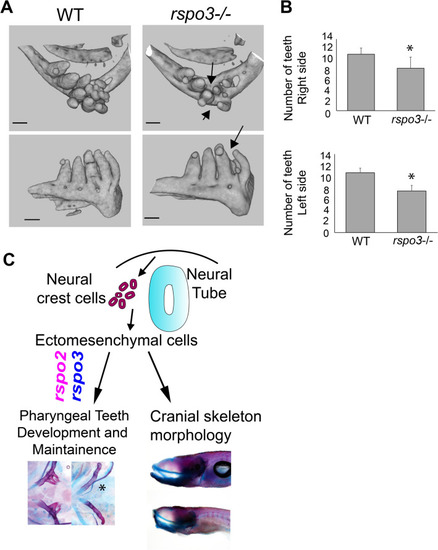
Synergistic effect of PHENOTYPE:
|
|
Synergistic effect of PHENOTYPE:
|
|
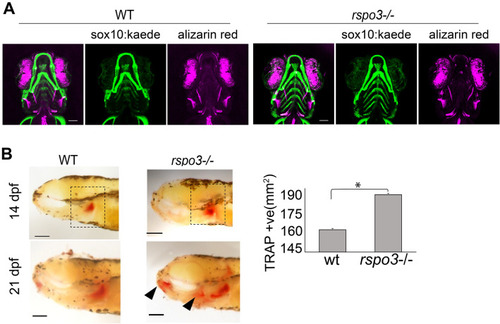
EXPRESSION / LABELING:
PHENOTYPE:
|
|
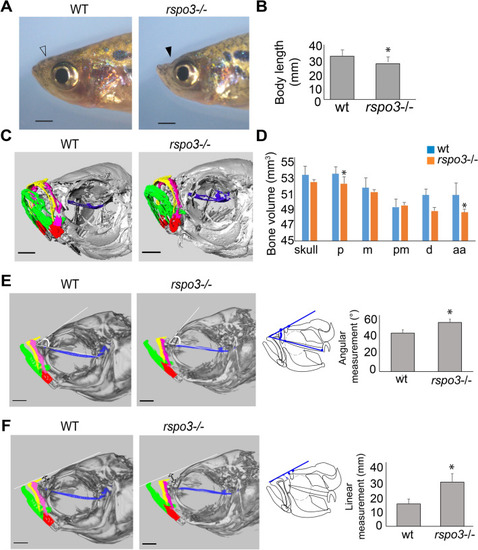
PHENOTYPE:
|
|
Adult PHENOTYPE:
|