- Title
-
Pcgf1 Regulates Early Neural Tube Development Through Histone Methylation in Zebrafish
- Authors
- Li, X., Ji, G., Zhou, J., Du, J., Li, X., Shi, W., Hu, Y., Zhou, W., Hao, A.
- Source
- Full text @ Front Cell Dev Biol
|
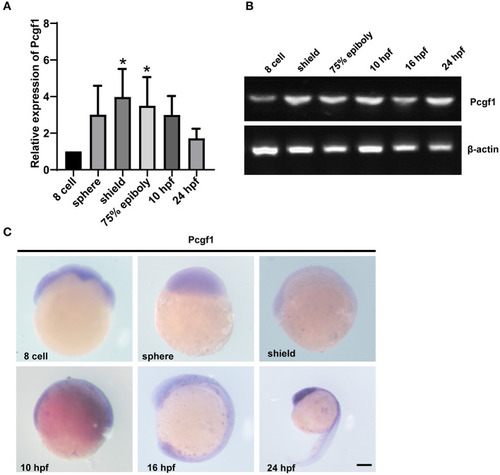
The expression pattern of Pcgf1 during zebrafish embryonic development. |
|
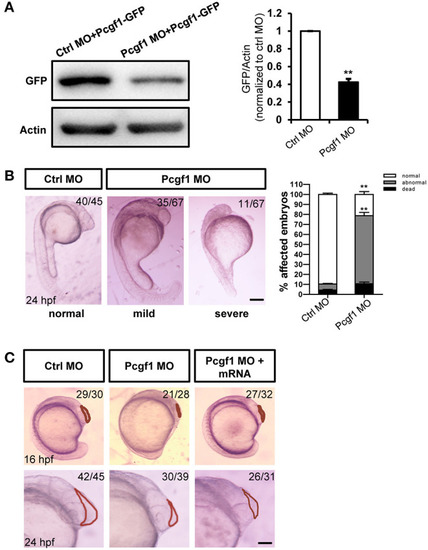
Zebrafish embryos displayed telencephalic malformations after knockdown of Pcgf1. PHENOTYPE:
|
|
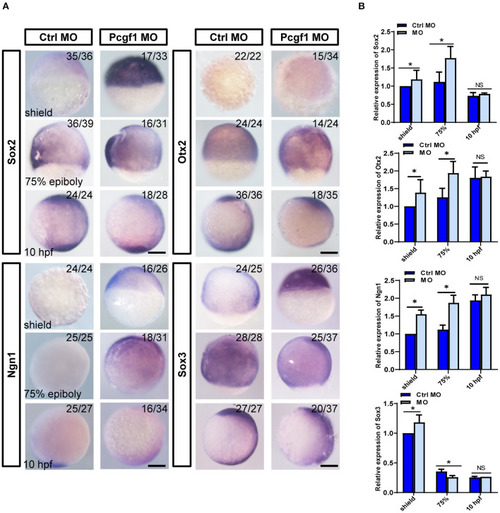
Effect of Pcgf1 on neural induction phase. (A) The expression levels of Sox2, Otx2, Ngn1, and Sox3 were detected by whole-mount in situ hybridization at neural induction phase (shield phase, 75% epiboly phase, and 10 hpf). Scale bar: 200 μm. (B) The expression of Sox2, Otx2, Ngn1, and Sox3 was analyzed by qPCR, and the expression of β-actin gene represented internal controls. Data represent the mean of at least three independent experiments ± SEM, *P < 0.05 vs. control MO. PHENOTYPE:
|
|
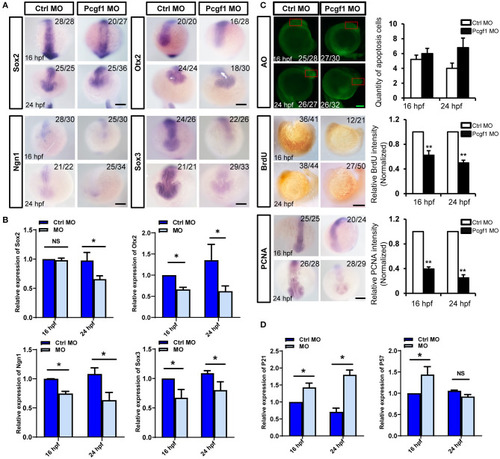
Effect of Pcgf1 on neural stem cells (NSCs) in neural tubes. EXPRESSION / LABELING:
PHENOTYPE:
|
|
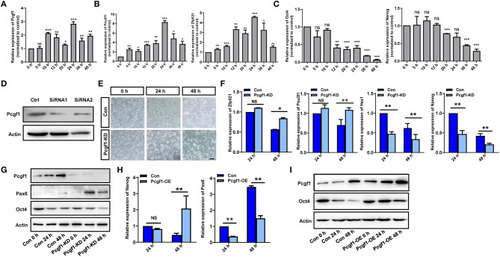
Pcgf1 had a positive role in maintaining the pluripotency of P19 cells. |
|
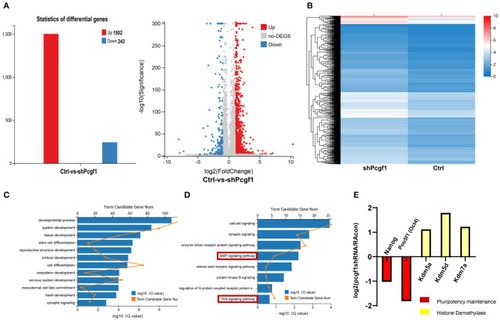
Pcgf1 regulated neural induction through an epigenetic mechanism in addition to signaling pathways. |
|
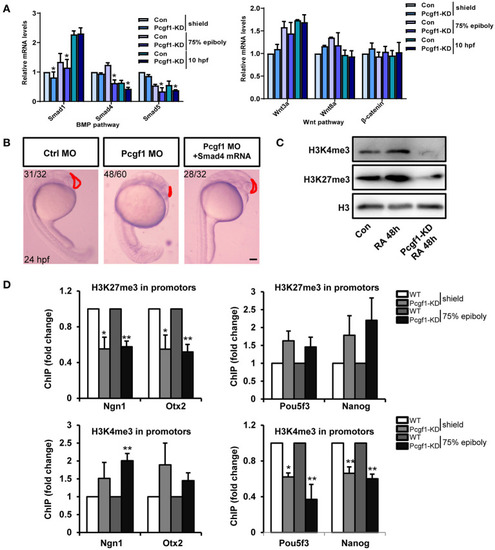
Pcgf1 regulated neural induction through histone methylation. |
|
Schematic representation of the effect of Pcgf1 on the neural induction and NSC self-renewal. |