- Title
-
Behaviorally consequential astrocytic regulation of neural circuits
- Authors
- Nagai, J., Yu, X., Papouin, T., Cheong, E., Freeman, M.R., Monk, K.R., Hastings, M.H., Haydon, P.G., Rowitch, D., Shaham, S., Khakh, B.S.
- Source
- Full text @ Neuron
|
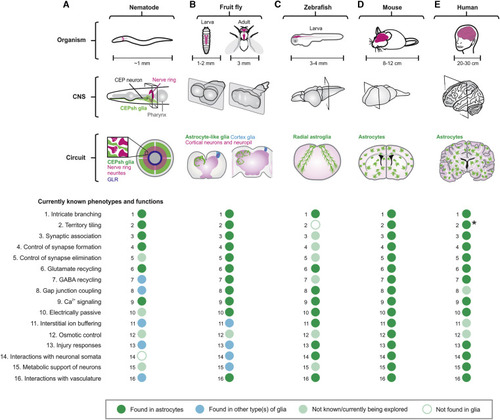
(A–E) The schematics illustrate the locations of CNS (gray), neuropil (purple), and astrocytes (green) in nematode (A), fruit fly (B), zebrafish (C), mouse (D), and human (E) at the level of organism, CNS, and circuit. Dot plots summarize that 16 well-defined cellular phenotypes/functions of astrocytes are found in astrocytes (green) or other type(s) of glia (blue), not known or currently being explored (light green), or not found in glia (white) in the relevant organism indicated. Some human astrocytes project long unbranched processes that cross cortical laminae (asterisk). |
|
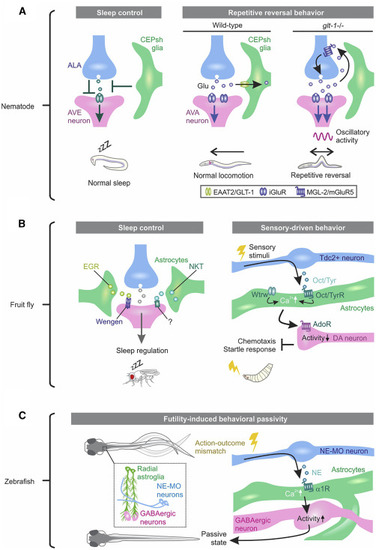
(A) Nematode CEPsh glia are required for normal sleep and locomotion. Left: the oscillatory activity of AVE neurons correlates with head retraction of the worm and regulates locomotion. ALA neurons are active during sleep and synaptically inhibit AVE. The working model from ablation experiments suggests that CEPsh glia tune the ALA-AVE synapse in proper behavioral state transition from wakefulness to sleep. Right: AVA neurons are a major class of interneurons driving backward locomotion. CEPsh glia take up glutamate (Glu) from the synaptic cleft at excitatory synapses onto AVA. Deletion of the glutamate transporter GLT-1 from CEPsh glia results in spillover of Glu from the synapses, activating presynaptic mGluR5 to cause repetitive excitation of AVA and reversal in worm locomotion. (B) Fly astrocytes regulate sleep and sensory-driven behavior. Left: the Drosophila TNF-α homolog Eiger (EGR), expressed in fly astrocyte-like cells (green), acts on Wengen, a receptor of EGR on neurons to regulate normal sleep. An astrocyte-enriched small secreted immunoglobulin (Ig)-domain protein, Noktochor (NKT), exhibits reduction and fragmentation in night sleep but not in day sleep. Right: upon sensory stimuli, Tdc2-expressing neurons release the neuromodulator tyramine (Tyr) or octopamine (Oct), the invertebrate analogs of NE, to increase Ca2+ in fly astrocytes in the ventral nerve cord. Waterwitch (Wtrw)/TRP channel also produce Ca2+ in the same types of astrocytes. Astrocyte Ca2+ signaling inhibits dopaminergic neuron firing via ATP/adenosine and is required for olfaction-driven chemotaxis and touch-induced startle responses. (C) Fish radial astroglia play causal roles in behavioral passivity triggered by futility. When fish recognize an accumulated unsuccessful attempt, noradrenergic neurons in the NE cluster of the medulla oblongata (NE-MO) become active, and released NE activates the α1-adenoceptor (AR) in radial astroglia. Radial astroglia Ca2+ signaling, in turn, enhances activity of GABAergic neurons in the lateral hindbrain to cause behavioral passivity. |
|
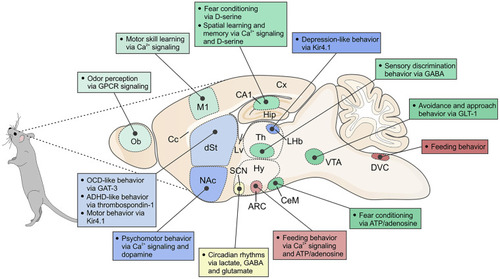
Schematic of a sagittal section of a mouse brain, depicting various regions and nuclei as well as associated behaviors that have been shown to be regulated by acute astrocytic mechanisms. Ob, olfactory bulb; Cx, cerebral cortex; M1, primary motor cortex; Lv, lateral ventricle; Cc, corpus callosum; dSt, dorsal striatum; NAc, nucleus accumbens; Hip, hippocampus; Th, thalamus; LHb, lateral habenula; Hy, hypothalamus; ARC, arcuate nucleus; SCN, suprachiasmatic nucleus; CeM, central amygdala; VTA, ventral tegmental area; DVC, dorsal vagal complex. In the text, we also consider sleep, but this is not illustrated here because it involves multiple brain nuclei. Furthermore, the cartoon does not include studies where behavioral alterations result over longer periods, such as following deletion of a critical gene within astrocytes or during development and aging. |