- Title
-
Nrl Is Dispensable for Specification of Rod Photoreceptors in Adult Zebrafish Despite Its Deeply Conserved Requirement Earlier in Ontogeny
- Authors
- Oel, A.P., Neil, G.J., Dong, E.M., Balay, S.D., Collett, K., Allison, W.T.
- Source
- Full text @ iScience
|
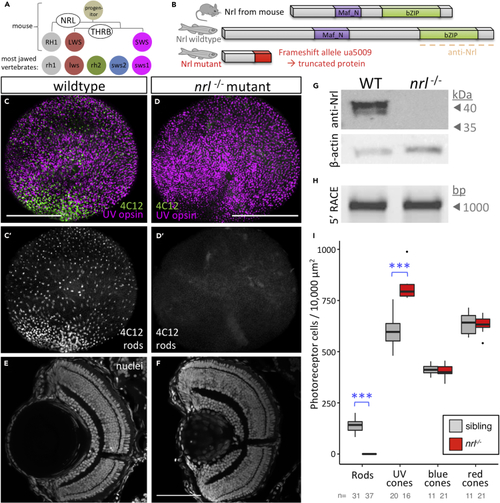
Nrl Is Conserved and Required for Rod Specification in Larval Zebrafish (A) Nrl is the master regulator of photoreceptor specification in mice, being both necessary and sufficient for rod photoreceptor development from progenitor cells. Elegantly simple models can account for cell fate specification and generating the full complement of photoreceptor types in mice (and all mammals studied) using only two factors, NRL and THRB (thyroid hormone receptor β), to generate rods, red cones, and blue cones expressing RH1, LWS, and SWS opsins, respectively. However, most vertebrates possess additional cone subtypes. (B) Zebrafish Nrl protein is recognizably similar to mammalian homologs. An (C and D) CRISPR-engineered null Nrl mutants lack rods in larval zebrafish, matching the phenotype of adult (E and F) Retina of (G) Nrl protein is not detectable in (H) Assessment of (I) Quantification of photoreceptor types in |
|
Zebrafish Nrl is Conserved and Sufficient to Induce Rod Photoreceptors in Zebrafish (A) Wild-type zebrafish larval retina in (B) Rods are more abundant in wild-type zebrafish by 6dpf. (B”) Expression of mouse Nrl reroutes cones to a rod cell fate (defined as GFP + cells in (C) Cellular morphology of UV cones |
|
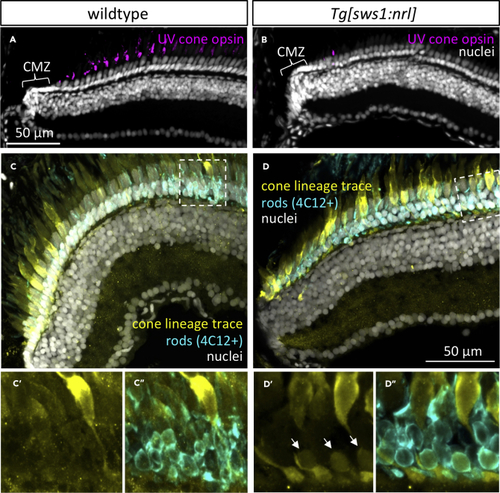
The Cone Cell Lineage Does Not Appreciably Contribute to Rod Production in Zebrafish but Inducing Ectopic Nrl Shows It has This Capacity (A) Adult zebrafish retina grows from proliferating cells in the ciliary marginal zone (CMZ), generating all cell types including regularly spaced UV cones. (B) Following ectopic expression of Nrl in differentiating UV cones, adult retina is mostly devoid of UV cones, except sparse newly born UV-opsin-positive cells near the CMZ. (A) and (B) are anti-UV opsin immunohistochemistry (magenta) with nuclear counterstain. (C) The adult zebrafish cone cell lineage gives rise to all cone cell types, and few other cell types are appreciably generated from that lineage, as detected by Cre-lox lineage tracing via a cone-transducin-α ( (D) The fate of UV-opsin-positive cells that are absent from mature retina in (B) includes their transmutation into rods. Note a subset of rod cells (arrows, identified as 4C12+ and with nuclei in the basal-most layer of the ONL) shows a history of EXPRESSION / LABELING:
PHENOTYPE:
|
|
Nrl is Dispensable for Rod Specification in Adult Zebrafish (A) Monitoring for appearance of GFP-positive rods during ontogeny of (B–D) Adult (E) Immunolabelling with rod-specific 4C12 and anti-UV-opsin confirms presence of rods and normal UV cones, respectively, in adult |
|
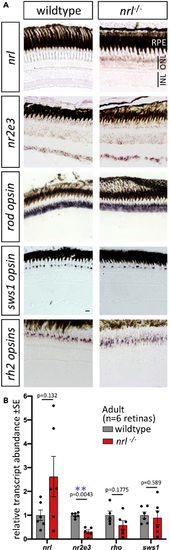
Adult Retina of Zebrafish (A) Gene expression determined by (B) Transcript abundance in adult neural retina determined by RT-qPCR confirms an increase in |
|
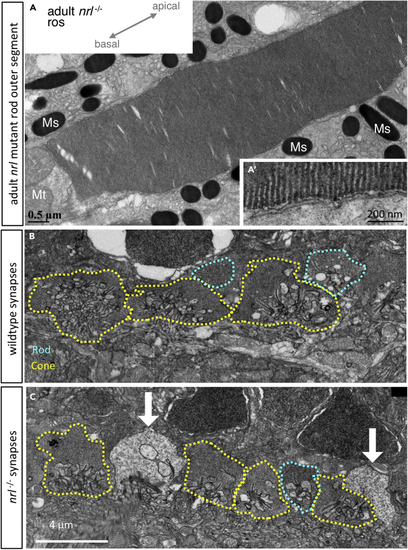
Rod Outer Segments of (A) Rods demonstrate the expected hairpin end of floating disks within the outer segment that are non-contiguous with the outer cell membrane (A′), diagnostic of rod cell identity (B) In wild-type adult zebrafish, photoreceptor synaptic terminals include rod spherules (teal dotted line) that are morphologically distinguishable from cone pedicles (yellow). (C) In PHENOTYPE:
|