- Title
-
P130Cas/bcar1 mediates zebrafish caudal vein plexus angiogenesis
- Authors
- Wisniewski, L., French, V., Lockwood, N., Valdivia, L.E., Frankel, P.
- Source
- Full text @ Sci. Rep.

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
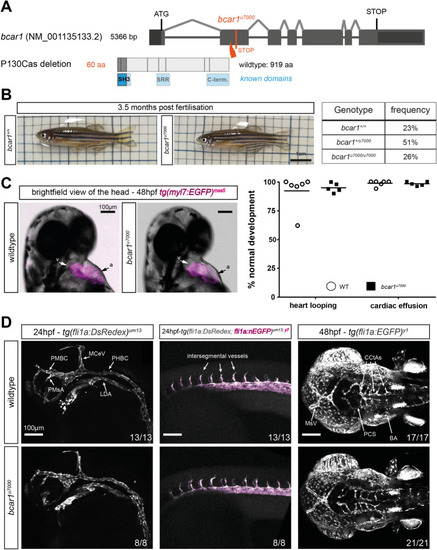
Loss of P130Cas does not induce lethality, major morphological defects, or defects in early arterial angiogenesis in zebrafish. ( |
|
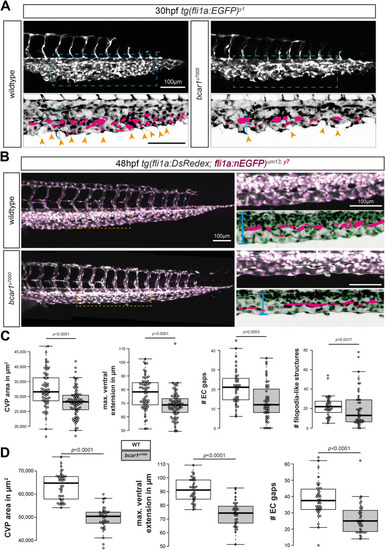
P130Cas is required for caudal vein plexus (CVP) angiogenesis. ( |
|
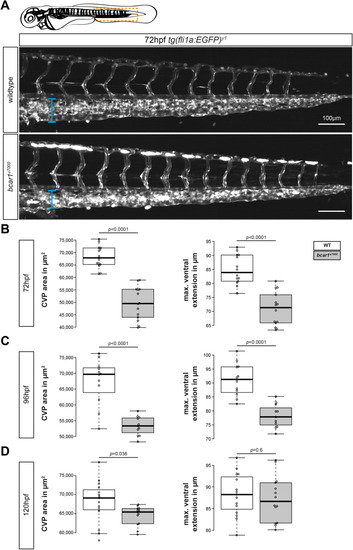
P130Cas deletion results in prolonged reduction of caudal vein plexus area. ( PHENOTYPE:
|
|
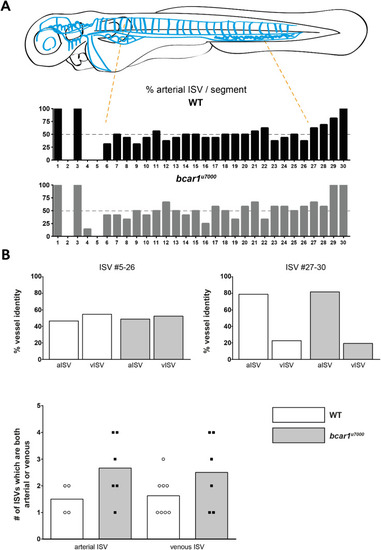
P130Cas is not required for venous intersegmental vessel formation at 72hpf. ( |
|
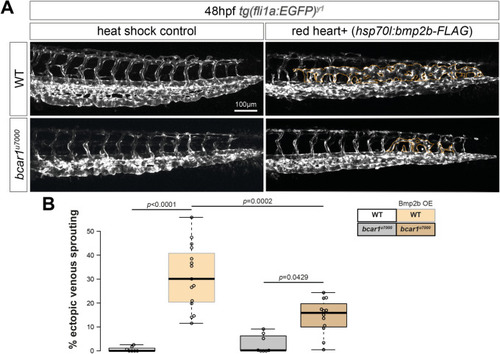
P130Cas mediates Bmp2b-induced formation of ectopic vessels. ( |
|
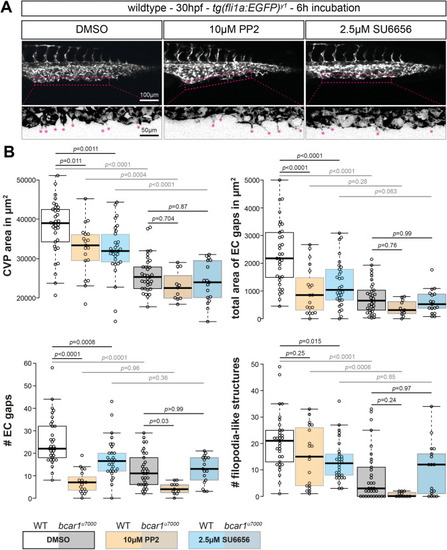
Src family kinases are required for caudal vein plexus formation. ( PHENOTYPE:
|