- Title
-
Genetic variation in GNB5 causes bradycardia by augmenting the cholinergic response via increased acetylcholine-activated potassium current (IK,ACh)
- Authors
- Veerman, C.C., Mengarelli, I., Koopman, C.D., Wilders, R., van Amersfoorth, S.C., Bakker, D., Wolswinkel, R., Hababa, M., de Boer, T.P., Guan, K., Milnes, J., Lodder, E.M., Bakkers, J., Verkerk, A.O., Bezzina, C.R.
- Source
- Full text @ Dis. Model. Mech.
|
|
|
|
|
|
|
|
|
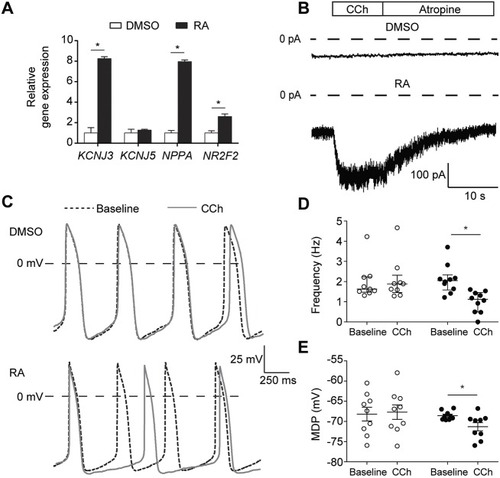
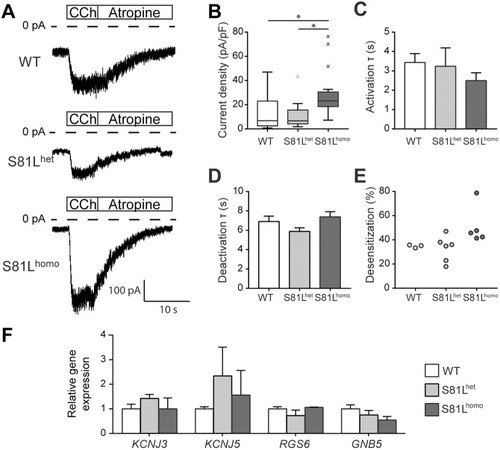
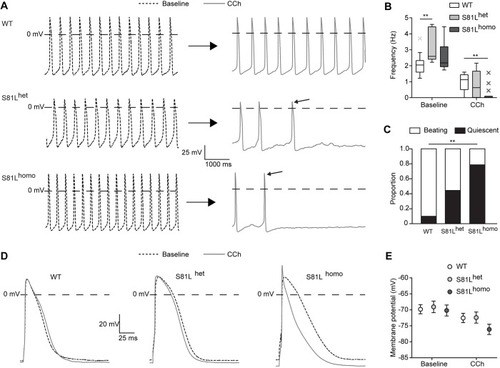
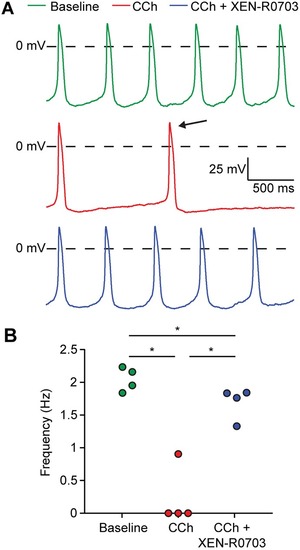
PHENOTYPE:
|
|
|
|
|