- Title
-
The Phenoxyphenol Compound 4-HPPP Selectively Induces Antiproliferation Effects and Apoptosis in Human Lung Cancer Cells through Aneupolyploidization and ATR DNA Repair Signaling
- Authors
- Liu, W., Wu, C.Y., Lu, M.J., Chuang, Y.J., Tsai, E.M., Leu, S., Lin, I.L., Ko, C.J., Chiu, C.C., Chang, W.T.
- Source
- Full text @ Oxid Med Cell Longev
|
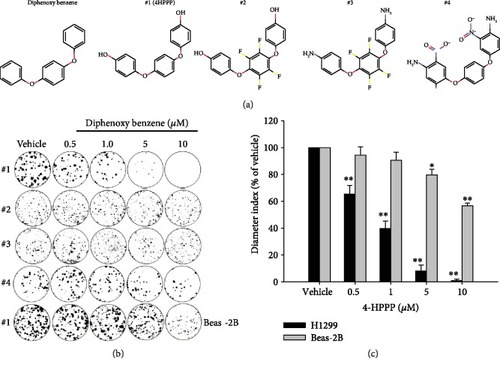
The inhibitory effect of compounds on the long-term proliferation of NSCLC cells. NSCLC H1299 cells and BEAS-2B human bronchial epithelial cells were treated with the indicated concentrations (from 0.5 to 10 |
|
4-HPPP induces apoptosis of NSCLC H1299 cells. The apoptosis induced by 4-HPPP was assessed using flow cytometry-based annexin V-PI staining. (a) H1299 cells were treated with 4-HPPP for the indicated time course. (b) The quantitative results of (a). |
|
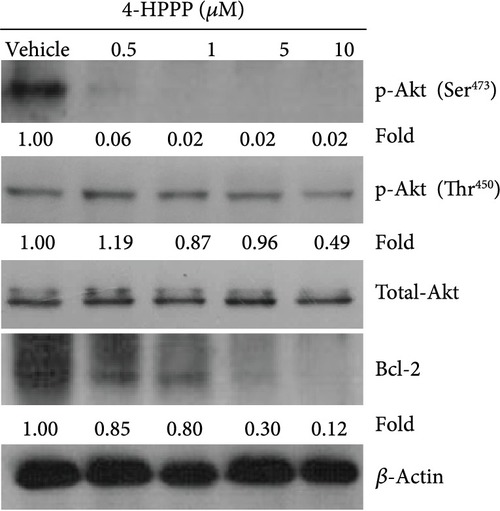
The effect of 4-HPPP on Akt phosphorylation changes in NSCLC cells. The phosphorylation changes at serine473 and threonine450 of Akt along with the prosurvival factor Bcl-2 were assessed using the Western blotting assay. |
|
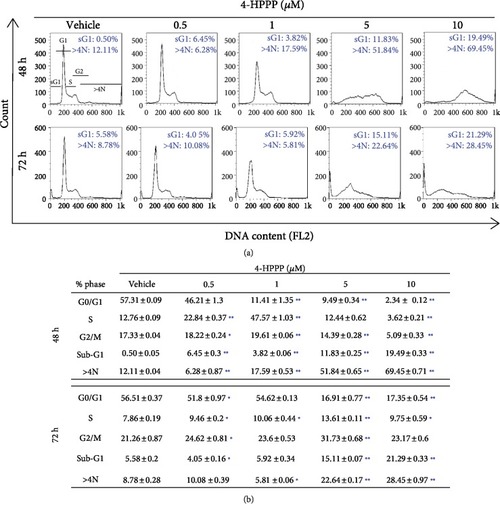
The effect of 4-HPPP on cell cycle progression. (a) The accumulation of sub-G1 and aneuploidy (N>4N) in NSCLC cells. H1299 cells were seeded and treated with different concentrations of 4-HPPP for 48 and 72 h. The treated cells were 70% ethanol-fixed and PI-stained and then subjected to a cell cycle distribution analysis by flow cytometry. Changes in cell cycle progression, the sub-G1 population, and aneuploidy following 4-HPPP treatment. (b) Quantitative analysis of cell cycle progression. ∗ |
|
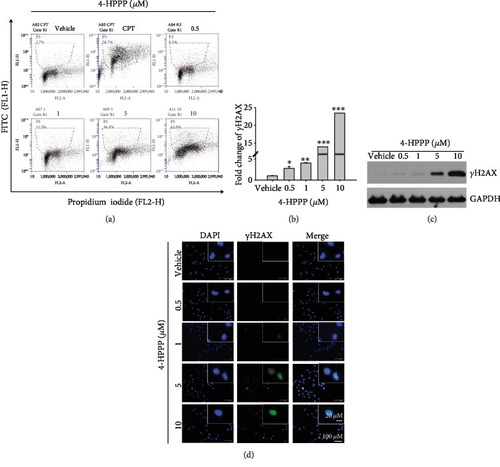
Assessment of DNA damage induced by 4-HPPP. (a) H1299 cells were administered the indicated concentrations of 4-HPPP for 48 h. Afterward, 4-HPPP-induced DNA damage was detected using a flow cytometry-based |
|
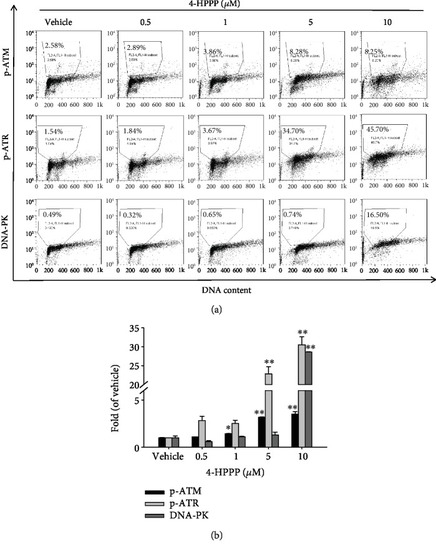
4-HPPP-induced activation of DNA damage markers in NSCLC cells. (a) H1299 cells were seeded and treated with 4-HPPP for 48 h. Afterward, the DNA damage induced by 4-HPPP was assessed by flow cytometry. The markers of DNA damage, including phosphor-ATR, phosphor-ATM, and DNA-PK, were determined. The results showed that 4-HPPP increased the phosphorylation of ATR and ATM and the protein level of DNA-PK in a dose-responsive manner. (b) The quantitative results of (a). ∗ |
|
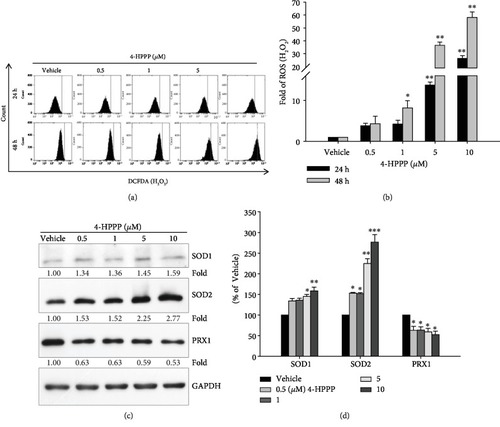
4-HPPP-induced changes in endogenous ROS and antioxidants in NSCLC cells. (a) H1299 cells were treated with the indicated concentrations of 4-HPPP for 24 h and 48 h. Afterward, intracellular levels of ROS were measured by the flow cytometry-based DCF-DA assay described in Materials and Methods. (b) Quantitative analysis of (a). (c) Changes in endogenous antioxidants SOD1, SOD2, and PRX1 in H1299 cells following 4-HPPP treatment by Western blotting. (d) Quantitative analysis of (c). ∗ |
|
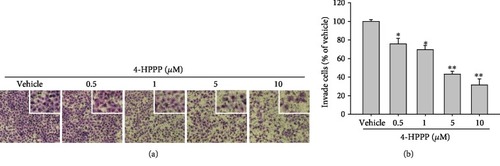
The effect of 4-HPPP on the motility of NSCLC cells. (a) H1299 cells were treated with the indicated concentrations of 4-HPPP for 18 h. Afterward, the cells were stained with 0.1% |
|
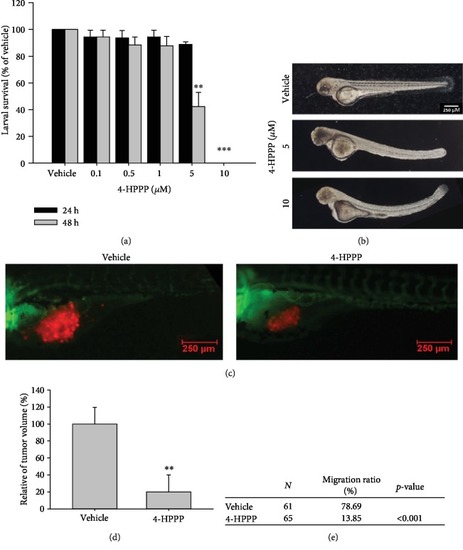
Effect of 4-HPPP on zebrafish-based xenografted NSCLC cells. (a) Survival rate of zebrafish larvae following 4-HPPP exposure. (b) The results showed that 5 |
|
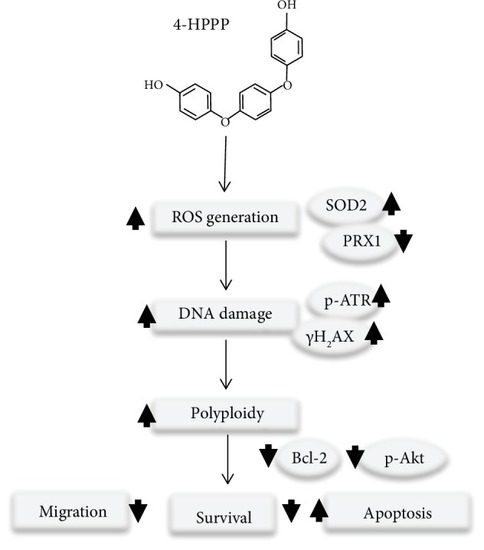
A proposed mechanism of 4-HPPP-induced antiproliferation and apoptosis in NSCLC cells through modulating the signaling of endogenous antioxidant systems, such as SOD2 and PRX1, causing DNA damage and high-grade aneuploidy. Eventually, the accumulation of DNA damage and hyperaneuploidization induce apoptosis in NSCLC cells. |