- Title
-
Estrogen accelerates heart regeneration by promoting the inflammatory response in zebrafish
- Authors
- Xu, S., Xie, F., Tian, L., Fallah, S., Babaei, F., Manno, S.H., Manno Iii, F.A.M., Zhu, L., Wong, K.F., Liang, Y., Ramalingam, R., Sun, L., Wang, X., Plumb, R., Gethings, L., Lam, Y.W., Cheng, S.H.
- Source
- Full text @ J. Endocrinol.
|
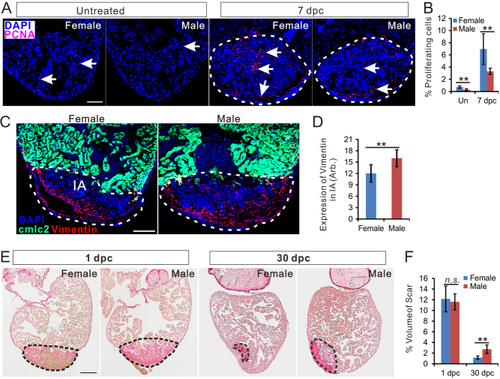
Zebrafish heart regeneration is sexually dimorphic. (A) PCNA immunofluorescence (red) in the heart of untreated female, untreated male, 7 dpc female (c) and 7 dpc male. Scale bars: 100μm. (B) Quantification of percentage of PCNA-positive cells (mean ± |
|
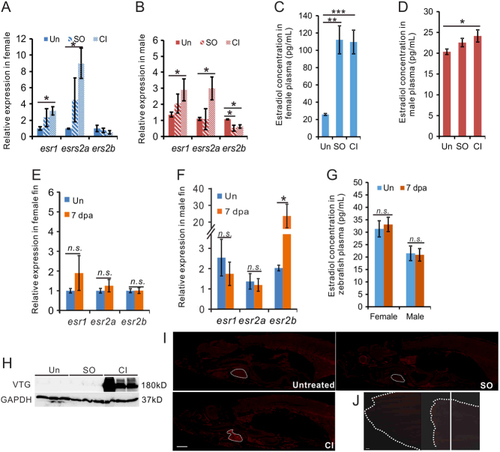
Cardiac damage increased estrogen expression in zebrafish. (A, B, C, D, E, F and G) qRT-PCR showing the expression of estrogen receptor genes in the heart (A, B) and fin (E, F) of zebrafish 7 days after CI; and plasma E2 concentration in zebrafish with heart (C, D) or fin (G) injury at 7 days. |
|
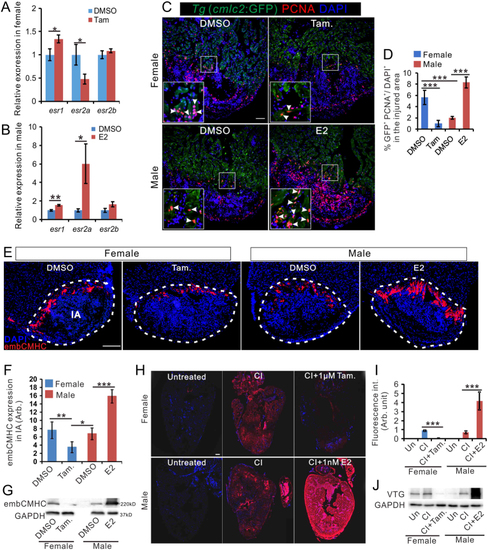
Estrogen promotes regeneration in zebrafish heart. (A and B) qRT-PCR showing the expression of estrogen receptor genes in the heart of female fish (A) and male zebrafish (B) with DMSO, tamoxifen or E2 treatment at 7 days after cryoinjury. |
|
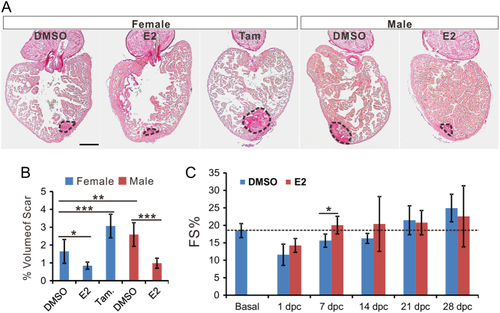
Estrogen accelerates scar reduction and promotes recovery of cardiac function. (A) Picrosirus red staining of the heart from female zebrafish treated with DMSO, E2 (1 nM) and tamoxifen (1 μM); and male zebrafish treated with DMSO and E2 (1 nM) at 30 dpc. Scale bar: 200 μm. (B) Quantification of scar volume from samples in panel A (marked by dash lines, mean ± |
|
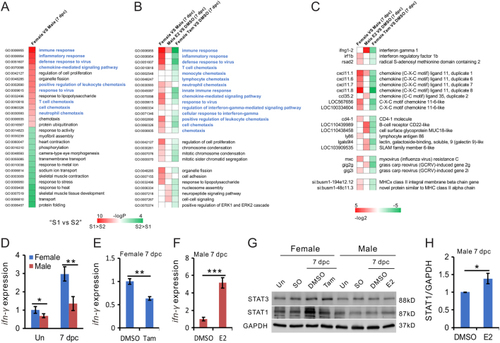
Estrogen induces inflammation in the injured zebrafish heart. A. Gene Ontogeny (GO) terms significantly enriched in genes differentially expressed in female vs male hearts at 7 dpc, |
|
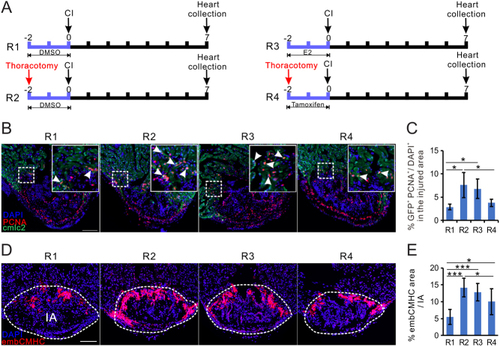
Estrogen preconditioning treatment promotes zebrafish heart regenerative process. (A) Experimental design of data presented in this figure. (B) PCNA immunofluorescence (red) in the heart of male |
|
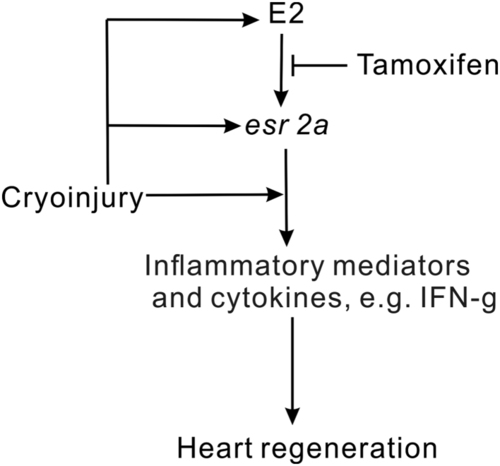
Proposed model estrogen role in zebrafish heart regeneration. Heart cryoinjury triggers the inflammation response and plasma estrogen upregulation. The increase of plasma estrogen induces expression of |