- Title
-
Type VII Secretion Substrates of Pathogenic Mycobacteria Are Processed by a Surface Protease
- Authors
- Burggraaf, M.J., Speer, A., Meijers, A.S., Ummels, R., van der Sar, A.M., Korotkov, K.V., Bitter, W., Kuijl, C.
- Source
- Full text @ MBio
|
MMARE11_28540 is required for the processing of LipY. (A) Secretion of LipY-OVA2 by |
|
PecA does not affect LipY surface localization and lipase activity. (A) Surface localization of LipY was measured on whole cells by flow cytometry for |
|
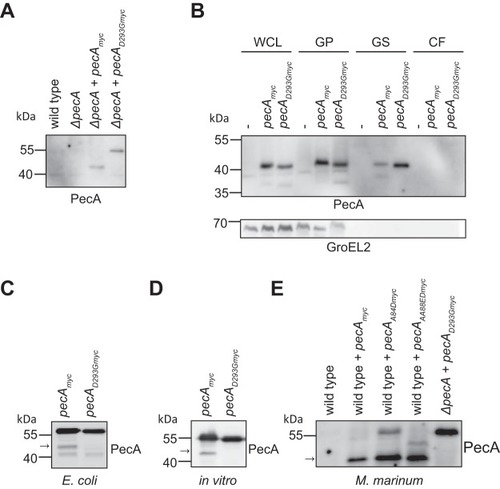
PecA is subjected to self-cleavage. (A) Expression of PecAmyc and PecAD293Gmyc in |
|
PecA is required for the processing of PE_PGRS proteins. Immunoblotting of whole-cell lysate from |
|
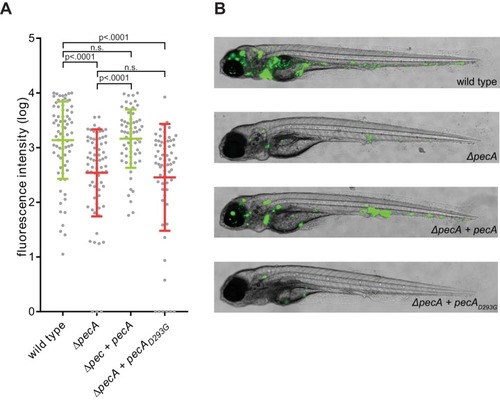
Deletion of PecA causes attenuation of virulence in an |
|
Model: PecA processes PE-PGRS proteins and is important for virulence. (A) PecA removes the PE domain of PE-PGRS proteins, including LipYtub and itself, at the surface of |