- Title
-
Myomesin is part of an integrity pathway that responds to sarcomere damage and disease
- Authors
- Prill, K., Carlisle, C., Stannard, M., Windsor Reid, P.J., Pilgrim, D.B.
- Source
- Full text @ PLoS One
|
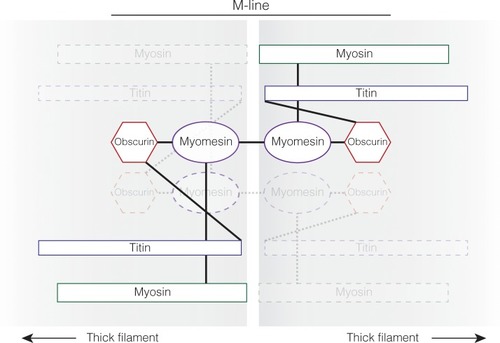
The M-line of the sarcomere diagraming the protein interactions between myosin thick filaments. The physical protein interactions through the M-line between antiparallel thick filaments are demonstrated by solid lines for one connection and faded dotted lines for the other connection between antiparallel thick filaments. Myosin and titin are incorporated into the M-line around the same time. This work suggests that myomesin is added next and requires both titin and myosin to be present for myomesin to be incorporated. Titin and myomesin together recruit obscurin, or obscurin-like 1, to the M-line [ |
|
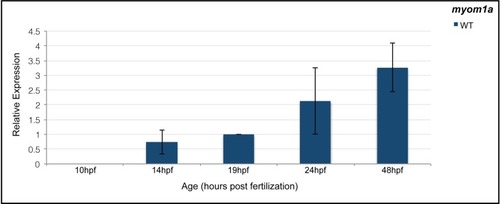
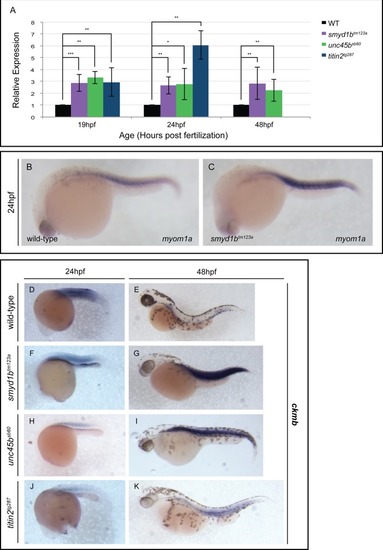
qPCR analysis of |
|
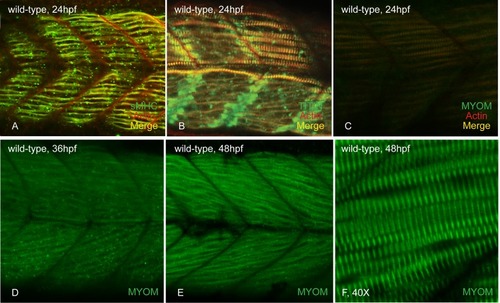
At 24 hpf, slow myosin (A) and titin (B) are incorporated and easily visible in the slow muscle fibers of wild-type embryos. Myomesin striations are observed in the parallel slow fibers of caudal somites (C). At 36 hpf, myomesin staining is seen in the developing fast fibers (D) and these striations become more organized and sharp as myogenesis continues at 48 hpf (E&F). |
|
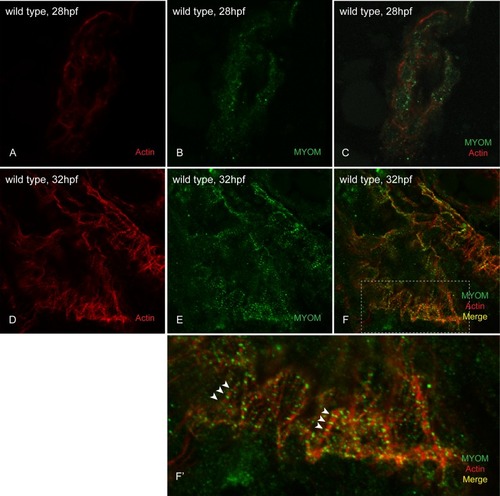
At 28hpf, we could not detect any incorporated myomesin into the sarcomeres of wild-type embryo hearts (A-C). However at 32hpf, myomesin striations are easily visualized in the hearts of wild-type embryos (D-F; white arrowheads in F’). |
|
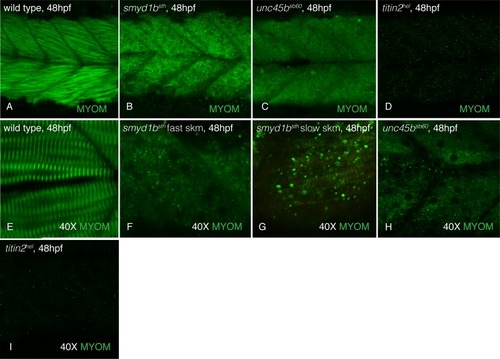
At 48hpf, myomesin is incorporated into the slow (perpendicular fibers) and fast (slanted fibers) muscle of wild-type siblings (A&E) while disorganized in |
|
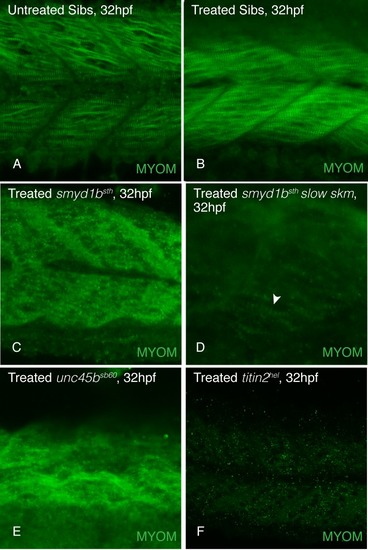
Embryos treated with tricaine to inhibit movement and contraction of skeletal sarcomeres from 18 to 32 hpf showed myomesin incorporates normally in untreated (A) and treated (B) wild-type siblings. Myomesin incorporation is absent in tricaine treated |
|
Zebrafish embryos treated with galanthamine (GAL) to induce muscle damage by hypercontraction from 28 to 48hpf demonstrated actin and myosin striations in both untreated (A&B) and treated (C&D) wild-type embryos; although GAL treated embryos exhibited fiber disorganization. Myomesin striations are visible in untreated wild-type embryos (E&F) while absent in GAL treated embryo muscle (G&H) even though actin striations, indicative of intact sarcomeres, are present (G’, white arrowheads; H’). qPCR analysis of |
|
qPCR analysis at 19, 24 and 48 hpf revealed a statistically significant up-regulation of EXPRESSION / LABELING:
PHENOTYPE:
|