- Title
-
Ciliary neurotrophic factor stimulates cardioprotection and the proliferative activity in the adult zebrafish heart
- Authors
- Bise, T., de Preux Charles, A.S., Jaźwińska, A.
- Source
- Full text @ NPJ Regen Med
|
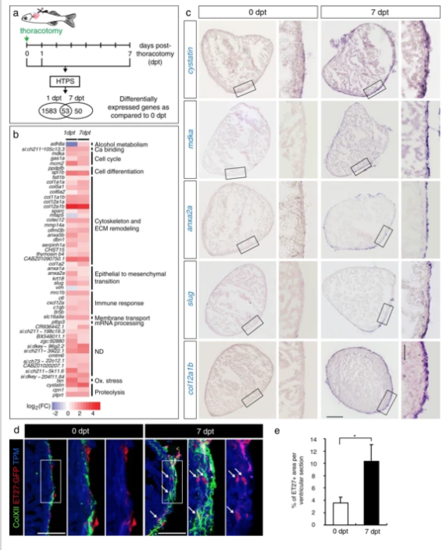
Transcriptional changes after thoracotomy suggest the activation of cardioprotective and EMT genes in the epicardium. a Experimental design. High throughput sequencing (HTPS) of ventricles collected at 0 (control), 1 and 7 days post-thoracotomy (dpt). For each group, RNA was extracted from a pool of 8 ventricles. The encircled numbers indicate differentially expressed genes at each time point. The middle number depicts differentially expressed genes common for both time points. b Heat-map representation of the common 53 differentially expressed genes at 1 and 7 dpt. Genes are grouped according to biological function. Fold changes are represented in log2 scale (blue: log2 < 0; red: log2 > 0). c In-situhybridization of ventricular transversal sections reveals upregulation of several candidate genes (purple) in the epicardial and sub-epicardial region at 7 dpt, compared to control hearts at 0 dpt. The frames indicate the part of the section that is magnified on the right side of each image. n ≥ 3 hearts. Scale bar for the whole section, 100 μm; for the magnified area, 50 μm. d Immunofluorescence staining of ventricular sections of transgenic fish ET27:EGFP(red) with antibodies against cardiac Tropomyosin (TMP, blue) and ColXII (green). At 0 dpt, ET27:EGFP + cells and ColXII are confined to the epicardium. At 7 dpt, ET27:EGFP + cells and ColXII expand and infiltrate the myocardium. Arrows indicate intramyocardial ET27:EGFP + cells. Scale bar, 50 μm. e Quantification of intramyocardial ET27:EGFP + area per ventricular section area. Superficial epicardial ET27 + cells were not included in measurements. n ≥ 3 hearts, 3 sections per heart each. *P < 0.05 with student’s t-test. Error bars represent standard error of the mean (s.e.m.) (This applies to the all subsequent figures) |
|
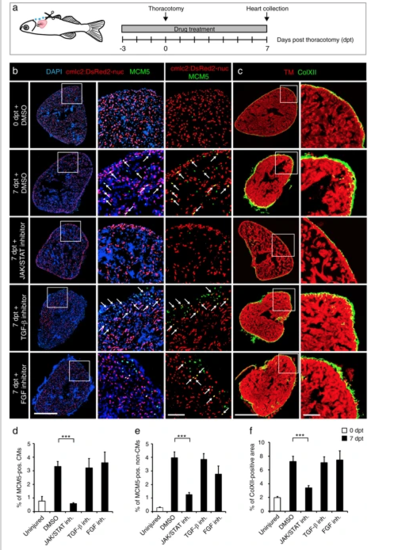
The cell-cycle entry and the deposition of ColXII after thoracotomy are dependent on the JAK/STAT3 pathway. a Experimental design. The requirement of different pathways for the increased CM mitotic activity and the ColXII deposition observed after preconditioning was tested at 7 dpt by using specific inhibitors of JAK/STAT (1 µM Ruxolitinib), TGF-β (20 µM SB431542) or FGF (10 µM PD173074) signaling. b, c Transversal sections of hearts treated with different drugs indicated at the left side. Scale bar for the whole section, 500 μm; for the magnified area, 100 μm. b Ventricle of transgenic fish cmlc2:DsRed2-nuc (red) immunostained for the G1/S-phase marker MCM5 (green). Some double positive cells are indicated with arrows. Treatment with Ruxolitinib markedly reduces cells proliferation in the ventricle. c Ventricle of wild type fish immunostained against Tropomyosin (red) and ColXII (green). In the presence of Ruxolitinib, intramyocardial ColXII is reduced. d Proportion of MCM5 + nuclei among cmlc2:DsRed2-nuc + nuclei. e Proportion of MCM5 + cmlc2:DsRed2-nuc-negative nuclei among DAPI + nuclei. f Proportion of the ColXII-positive area within the surface of ventricular section. n ≥ 4 hearts; ≥ 2 sections per heart; ***P < 0.001 PHENOTYPE:
|
|
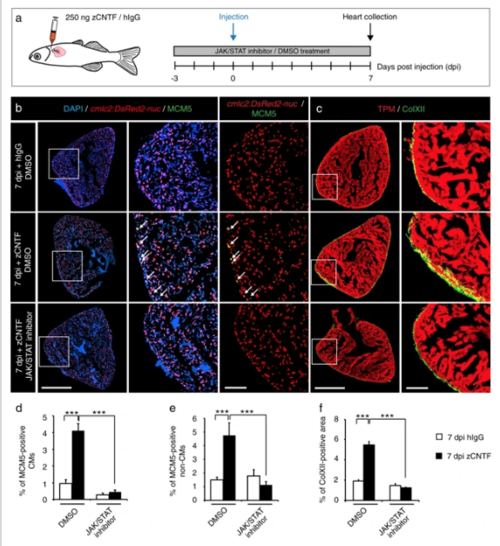
Single injection of zCNTF elevates the mitotic activity and ColXII deposition. a Experimental design to assess effects of a single intrathoracic injection of zCNTF in the presence of the JAK/STAT inhibitor, 1 µM Ruxolitinib, at 7 dpi. b, c Immunofluorescence staining of heart sections. Scale bar for the whole section, 500 μm; for the magnified area, 100 μm. bTransversal heart sections of transgenic fish cmlc2:DsRed2-nuc (red) to demarcate CM nuclei, immunostained with the G1/S-phase marker MCM5 (green) display enhanced CM proliferation (arrows) after zCNTF injection in the absence of the JAK/STAT inhibitor. cTransversal heart sections of wild type fish immunostained with of ColXII (green) and Tropomyosin (red). Intramyocardial ColXII is increased in zCNTF-injected hearts. JAK/STAT inhibition abolished this effect. d Proportion of MCM5-positive cells among cmlc2:DsRed2-nuc/DAPI-positive CM nuclei. e Proportion of MCM5 + cmlc2:DsRed2-nuc-negative nuclei among DAPI + nuclei. f Proportion of the ColXII-positive area within the surface of ventricular section. n ≥ 4 hearts; ***P < 0.001 |
|
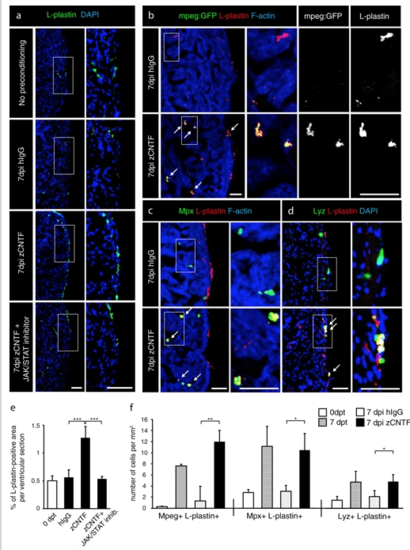
Exogenous zCNTF recruits leucocytes into the zebrafish uninjured heart. a–dImmunofluorescence staining of heart sections from different conditions as indicated at the left side of the images. a Hearts of wild type fish stained for L-plastin (green) and DAPI (blue). Single injection of zCNTF results in higher accumulation of L-plastin in the heart at 7 dpi. This effect is abolished in the presence of the JAK/STAT inhibitor, 1 µM Ruxolitinib. Scale bars, 100 μm. b Hearts of transgenic fish mpeg1:EGFP (green) stained for L-plastin (red) and F-actin (Phalloidin, blue). The number of mpeg1:EGFP/L-plastin-expressing cells is increased at 7 days after zCNTF injection. Some double positive cells are indicated with arrows. Scale bers 50 μm. c, d Ventricles of wild type fish stained for Mpx (c, green) or Lyz (d; green) and F-actin (Phalloidin, blue). The number of double positive leucocytes is increased at 7 days after zCNTF injection (arrows). Scale bars, 50 μm. e Quantification of L-plastin + area in ventricular sections. f Quantification of cells expressing mpeg1:EGFP, Mpx and Lyz normalized to the ventricle area at different conditions. n ≥ 4 hearts; ≥ 2 sections per heart; *P < 0.05; **P < 0.01; ***P < 0.001 |
|
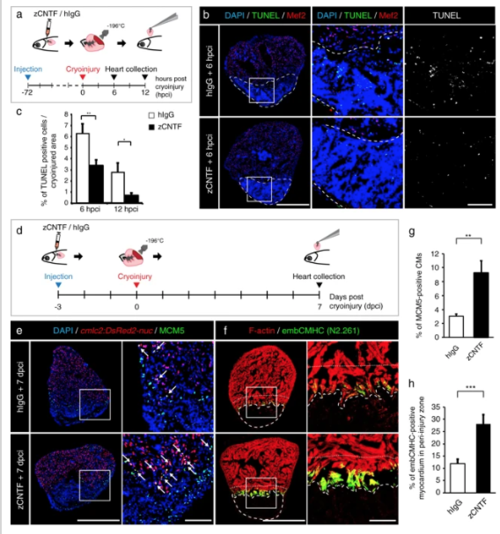
Administration of zCNTF reduces apoptosis and boosts initiation of heart regeneration after cryoinjury. a Experimental design for the apoptosis assay. zCNTF was injected into the pericardial cavity 72 h before cryoinjury. Hearts were collected at 6 and 12 h post-cryoinjury (hpci). b Transversal heart sections at 6 hpci stained for apoptotic cells using the TUNEL assay (green) and a myocyte nuclear marker Mef2 (red). Cryoinjured zone is encircled with a dashed line. Scale bar for the whole section, 500 μm; for the magnified area, 100 μm. cQuantification of TUNEL-positive nuclei at 6 and 12 hpci, in hearts injected with control proteins and zCNTF. n ≥ 4 hearts; ≥ 2 sections per heart; *P < 0.05; **P < 0.01. d Experimental design for assessment of the initiation of regeneration. zCNTF was injected into the pericardial cavity at 3 days before cryoinjury (dpci). Hearts were collected at 7 dpci. e, fImmunofluorescence staining of heart sections. Scale bar for the whole section, 500 μm; for the magnified area, 100 μm. e Transversal heart sections of transgenic fish cmlc2:DsRed2-nuc immunstained for MCM5 (green) display an enhanced number of proliferating CMs (arrows) after zCNTF injection. f Transversal heart sections of zCNTF/hIgG injected wild type fish immunostained for embCMHC (N2.261, green) and F-actin (red) comprise more abundant expression of embCMHC in the peri-injured ventricle within a distance of 100 μm from the wound tissue, which is delineated with a dotty line. g Quantification of MCM5-positive cardiac nuclei in hearts injected with control proteins and zCNTF. n ≥ 4 hearts; ≥ 2 sections per heart; *P < 0.05; **P < 0.01. h Proportion of the embCMHC-positive myocardium within the 100 μm peri-injury zone in hearts injected with control proteins and zCNTF. n ≥ 4 hearts; ≥ 2 sections per heart; ***P < 0.001 |
|
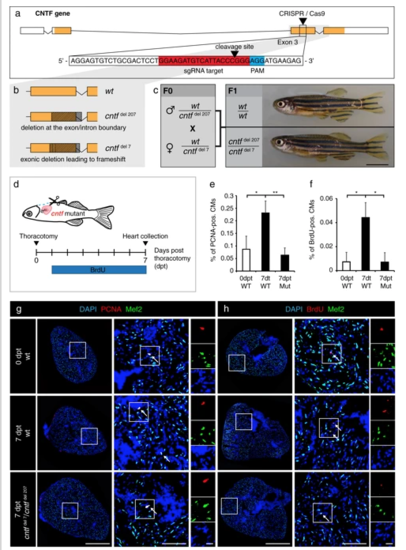
Cntf mutants fail to enhance CM proliferation after thoracotomy. a Schematic drawing of the cntf locus containing 4 exons. UTR (white); translated sequences (orange boxes). The sequence of 154-174 nucleotides (in red) flanking PAM sequence (in blue) in the 3rd exon was targeted by the CRISPR/Cas9-sgRNA RNP complex. b Schematic drawing of the two deletions induced by CRISPR/Cas9. cntfdel207 contains a deletion of 207 bp in the 3rd exon spanning the exon/intron boundary. cntfdel7 comprises a deletion of 7 bp in the middle of the 3rd exon leading to a frameshift and a premature stop codon. c Genotypes and images of wild type and CNTF mutant siblings. F0 mosaic heterozygous candidates were crossed to obtain F1 trans-heterozygous mutants, which are viable without visible phenotype. Scale bar, 5 mm. dExperimental design. e Quantification of PCNA-positive cells among Mef2/DAPI-positive CM nuclei. n ≥ 3 hearts; ≥ 3 sections per heart; *P < 0.05, **P < 0.01. f Quantification of BrdU-positive cells among Mef2/DAPI-positive CM nuclei. n ≥ 3 hearts; ≥ 3 sections per heart; *P < 0.05. g, h Transversal heart sections of wt and cntf mutant fish at 7 dpt, immunostained against Mef2 (green, a myocyte nuclear marker) and cell proliferation markers, PCNA (g, red) or BrdU (h, red). All nuclei are labeled with DAPI (blue). Arrows indicate some proliferating CMs. Scale bar for the whole section, 500 μm; for the magnified area, 100 μm; for the zoom of magnified area, 20 μm |
|
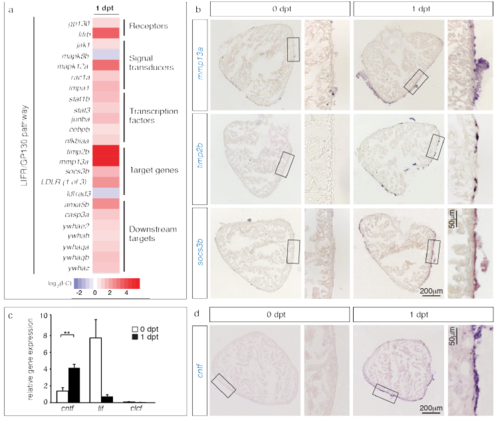
Components of the LIFR/GP130 pathway are transcriptionally upregulated after preconditioning. (a) Heat map representation of 20 differentially expressed genes at 1 dpt that contribute to the LIFR/gp130 pathway. Fold changes are represented in log2 scale of fold change (blue: log2 < 0; red: log2 > 0). (b) In-situ hybridization of ventricular sections demonstrates upregulation of several components of the LIFR/GP130 pathway at 1 dpt. n ≥ 3 hearts. (c) Detection of putative LIFR/gp130 ligands at 1 dpt by qRT-PCR. The normalized gene expression was calculated relative to β-actin. n ≥ 3 samples prepared of 10 ventricles. **P < 0.01 with student’s t-test. (d) In-situ hybridization of ventricular sections demonstrates upregulation of cntf transcription at the periphery of the ventricle, at 1 dpt. n ≥ 3 hearts. |
|
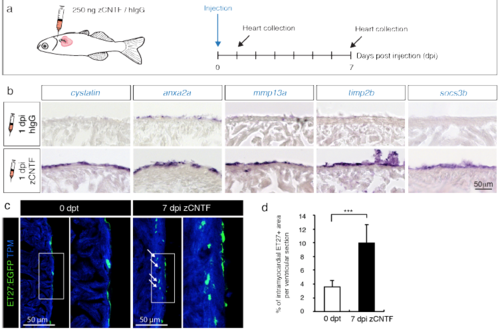
Exogenous zCNTF induces expression of preconditioning genes and activates the epicardium. (a) Experimental design to assess effects of a single pericardial injection of zCNTF on gene expression and the epicardium. (b) In-situ hybridization of ventricular sections with different probes at 1 day post-injection (1 dpi) of control proteins (hIgG) and zCNTF. Several candidate genes identified in transcriptomic analysis are upregulated on the heart surface on the next day following zCNTF injection. n ≥ 3 hearts, 3 sections per heart each. (c) Immunofluorescence staining of ventricular sections of the transgenic fish ET27:EGFP (green) with antibodies against cardiac Tropomyosin (TPM, blue). At 0 dpt, ET27:EGFP+ cells are confined to the epicardium. At 7 dpi, ET27:EGFP+ cells expand and infiltrate the myocardium (arrows). (d) Quantification of intramyocardial ET27:EGFP+ area per ventricular section area. Superficial epicardial ET27+ cells were not included in measurements. n ≥ 3 hearts, 3 sections per heart each. *** P<0.001with student’s t-test. |
|
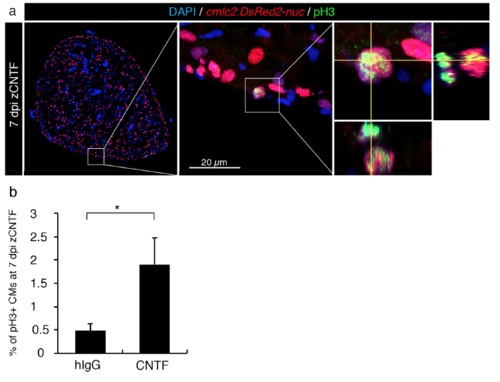
Injected zCNTF increases mitosis in the uninjured zebrafish heart. (a) Immunofluorescence staining of ventricular sections of the transgenic fish cmlc2:DsRed2-nuc (red) with antibodies against phospho-Histon H3 (pH3, green) of an intact heart at 7 dpi with zCNTF. The middle panel represents the higher magnification of the framed area in the left panel. The image on the right side shows a mitotic CM. Orthogonal projections along the yellow planes demonstrate a dividing CM. (b) Quantification of pH3-positive CM at 7 dpi with hIgG and zCNTF. n ≥ 8 hearts, 5 sections per heart each. * P<0.05 with student’s t-test. |
|
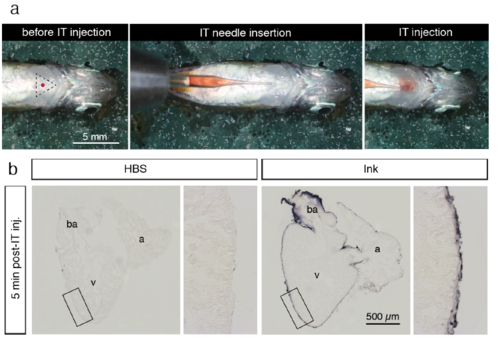
Intrathoracic microinjection procedure. (a) Photographs of the microinjection procedure under the stereomicroscope. Before intrathoracic (IT) injection, an anesthetized fish was positioned with the ventral side up on a moist sponge. The tip of the pulled glass needle with a 2.5 μl solution was inserted under the skin of the thorax. The solution was slowly injected into the pericardial activity with a caution not to puncture the heart. (b) Histological sections of the zebrafish heart for a validation of accuracy of the microinjection procedure. The fish were euthanized and IT-injection was performed using 2.5 μl Hank’s buffer (HBS) or purple ink. At 5 min post injection, the hearts were collected, fixed and sectioned. Injected ink labeled the surface of the ventricle (v), atrium (a) and bulbus arteriosus (ba), suggesting efficient spreading of the injected solution throughout the pericardial cavity. No dye was observed inside the organ, demonstrating that the needle did not puncture the heart during injection. |