- Title
-
Maternal Huluwa dictates the embryonic body axis through β-catenin in vertebrates
- Authors
- Yan, L., Chen, J., Zhu, X., Sun, J., Wu, X., Shen, W., Zhang, W., Tao, Q., Meng, A.
- Source
- Full text @ Science
|
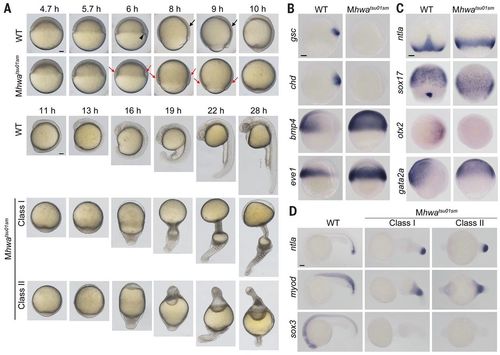
Mhwatsu01sm mutant phenotype. (A) Morphogenesis of live wild-type (WT) and mutant embryos. The black arrowhead and arrows indicate the dorsal organizer and the thickening body axis, respectively; the red arrows in mutants indicate the thicker blastodermal marginal zone. See also movie S1. (B and C) Germ layer marker expression detected by WISH during gastrulation. Orientation: ntla and sox17, dorsal views with animal pole to the top at 75% epiboly stage; others, lateral views (gsc, chd, bmp4, eve1, and gata2a) or animal-pole view (otx2) with dorsal to the right. (D) Marker expression at 24 hpf. Lateral views with anterior to the left. Scale bars, 100 μm. |
|
Identification and verification of tsu01sm mutant gene. (A) Top: Illustration of the zebrafish hwa wild-type and tsu01sm mutant alleles. Arrowheads indicate the positions of PCR primers. The green arrow indicates the translation start site. Bottom: Electrophoretic result of PCR products in wild-type, heterozygous, and homozygous mutant embryos. M, molecular weight markers. (B) hwa mRNA detection by WISH (left) and by qRT-PCR (right). bb, Balbiani body in stage I oocyte. For qRT-PCR, oocytes are a pool of stage I–III oocytes and eggs are squeezed from females. The relative hwa mRNA levels are averages (±SEM) from three independent experiments, normalized to eif4g2a levels. ***P < 0.001. Scale bars, 100 μm. (C) Mhwatsu01sm mutant embryos form the body axis or are dorsalized by hwa overexpression. Left: Morphology of classified embryos at 24 hpf. Right: Ratios of embryos in different classes. (D) Induction of secondary body axis by ectopic hwa mRNA overexpression. One blastomere of 32-cell stage Tg(gsc:EGFP) transgenic embryos or two opposite blastomeres of 32-cell stage Tg(gsc:EGFP);Mhwatsu01sm mutant embryos were injected with 50 pg of hwa and 50 pg of mCherry mRNAs, as illustrated at left. Embryos were observed dorsally (top) or laterally (bottom) at 6 hpf under a fluorescence dissection microscope, and those with two dorsal organizers (EGFP-positive) were observed again at 24 hpf. The ratio of embryos with double organizers is indicated. Scale bars, 100 μm. (E) Generation of a hwa mutant allele by Cas9 knockout. Top: Illustration of the mutant allele with an underlined premature stop codon. Bottom left: A group of Mhwatsu32c mutant embryos at 24 hpf. Bottom right: Alteration of the dorsal markers gsc and chd and the ventral marker eve1 at shield stage; scale bar, 100 μm. |
|
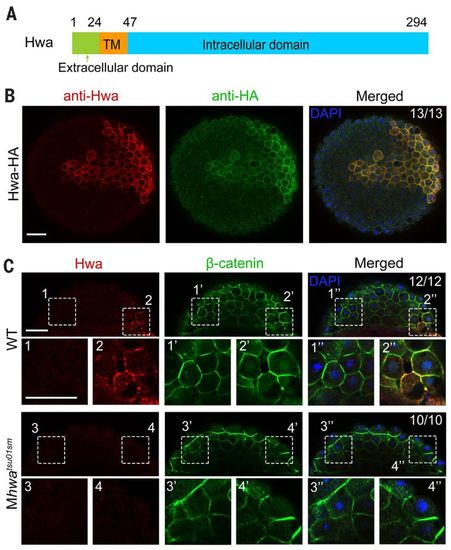
Hwa is localized on the plasma membrane of dorsal blastomeres. (A) Illustration of zebrafish Hwa protein structure. Positions of residues at the start of the domain are indicated except the last residue position. (B) Detection of exogenous Hwa-HA by immunostaining using anti-Hwa antibody: 50 pg of hwa-HA mRNA was injected into one blastomere of eight-cell stage Mhwatsu01sm mutant embryos, and the embryos were collected at 4 hpf for immunostaining with anti-HA and anti-Hwa antibodies. Nuclei were also stained with 4′,6-diamidino-2-phenylindole (DAPI). Embryos were imaged by confocal microscopy in animal pole view. Note that exogenous Hwa-HA is enriched on the plasma membrane. Scale bar, 50 μm. (C) Location of endogenous Hwa protein. Zebrafish wild-type and Mhwatsu01sm mutant embryos were collected at the 512-cell stage for coimmunostaining of endogenous Hwa and β-catenin. Embryos were imaged by confocal microscopy and are shown in lateral view with the animal pole to the top. In all 12 wild-type embryos with β-catenin in nuclei of the dorsal blastomeres, endogenous Hwa protein is enriched on the plasma membrane in the same region (top panel). In Mhwa mutants (bottom panel), Hwa signal is absent on the plasma membrane and no blastomeres have nuclear β-catenin. For each embryo, two numbered areas are enlarged for better view. The ratio of embryos with the representative pattern is indicated. Scale bars, 50 μm. |
|
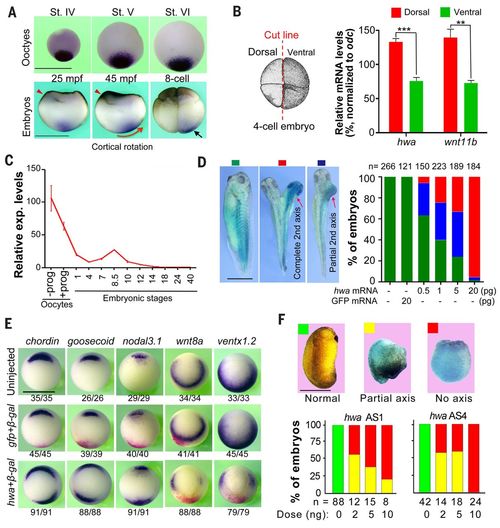
Roles of Xenopus hwa during embryonic body axis formation. (A) Expression of hwa in oocytes and embryos by WISH detection. Red arrowheads indicate the sperm entry site. St, stage; mpf, minutes post-fertilization. The red arrow shows the cortical rotation direction; the black arrow indicates asymmetrically located hwa transcripts. (B) Quantification of hwa transcripts in two dorsal and two ventral blastomeres at four-cell stage by qRT-PCR. Left: Illustration of cutting of embryos. Right: qRT-PCR result showing average levels from three technical repeats (±SEM) normalized to odc level. The dorsal marker wnt11b serves as an indicator. **P < 0.01, ***P < 0.001. (C) Quantification of hwa mRNA levels by qRT-PCR at the indicated stages; prog, progesterone. hwa expression level is averaged from technical repeats (±SEM) and normalized to odc level at the indicated stages. (D) Induction of secondary axis by hwa in Xenopus embryos. One ventral blastomere of four-cell stage embryos was injected with different doses of hwa-myc or 20 pg of GFP mRNA plus 200 pg of β-gal mRNA and observed at stage 32. Left: Classes of embryos. Right: Ratios of classified embryos. (E) Organizer and ventral marker expression patterns detected by WISH at stage 10 (vegetal view). One ventral blastomere of four-cell stage embryos was injected with 20 pg of hwa or 100 pg of GFP mRNA plus 200 pg of β-gal mRNA. The ratio of embryos with the representative pattern is indicated. (F) Maternal knockdown effect of hwa. Oocytes were injected with different doses of antisense oligodeoxynucleotides (AS1 or AS4) and the resulting embryos were observed and categorized at stage 24. All scale bars, 1 mm. |
|
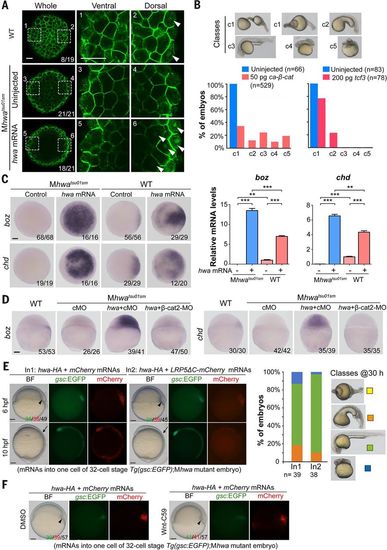
hwa can function through β-catenin in a Wnt ligand/receptor-independent manner in zebrafish embryos. (A) Immunostaining of β-catenin. After injection of 50 pg of hwa mRNA at one-cell stage, embryos were immunostained for β-catenin at 512-cell stage and observed by confocal microscopy. Whole embryos are viewed from the animal pole; two opposite marginal areas are viewed at a higher magnification. The ratio of embryos with the representative pattern is indicated. (B) Recovery of the body axis in Mhwatsu01sm embryos by ca-β-catenin or tcf3 overexpression. Embryos were injected at one-cell stage and observed at 24 hpf. The embryos are categorized into five classes (top panel); ratios are shown below. (C) hwa overexpression induces the expression of the β-catenin target genes boz and chd. Wild-type or Mhwatsu01sm embryos were injected with 50 pg of hwa-HA mRNA at the one-cell stage and collected at 4 hpf for WISH or qRT-PCR analysis. Left: WISH results (animal-pole views). Right: qRT-PCR results showing averages from three independent experiments (±SEM). **P < 0.01, ***P < 0.001. (D) hwa overexpression fails to induce boz and chd in β-cat2 morphants. Wild-type or Mhwatsu01sm embryos were injected with 50 pg of hwa-HA mRNA together with 20 ng of control MO (cMO) or β-cat2-MO at the one-cell stage and subjected to WISH at 4 hpf. Embryos are laterally viewed. (E and F) Inhibition of Wnt ligand/receptor signaling has no effect on hwa’s organizer/axis-inducing activity. (E) One blastomere of Tg(gsc:EGFP);Mhwatsu01sm embryos at about 32-cell stage was injected with 50 pg of hwa plus 200 pg of mCherry (In1) or LRP5ΔC-mCherry mRNA (In2). Left: Embryos are shown at 6 and 10 hpf. Embryo ratios are expressed as GFP+/mCherry+/total. Right: Bar graph showing ratios of classes of 30-hpf embryos growing from GFP+ 6-hpf embryos. Note that the class I mutant phenotype did not show in any injected embryos and served only for comparison. (F) Tg(gsc:EGFP);Mhwatsu01sm embryos injected with hwa and mCherry mRNAs as in (E) were incubated in DMSO or 20 μM Wnt-C59. The organizer and body axis are indicated by arrowheads and arrows, respectively. Scale bars, 10 μm (A), 100 μm (all others). |
|
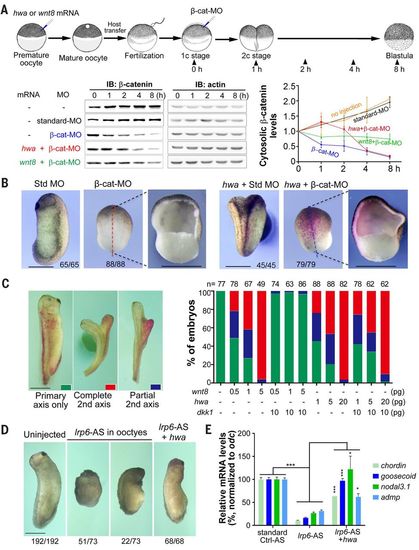
hwa can function through β-catenin in a Wnt ligand/receptor-independent manner in Xenopus embryos. (A) hwa or wnt8 overexpression delays cytosolic β-catenin degradation. Top: Illustration of experiment design (dosage: hwa or wnt8 mRNA, 200 pg; β-catenin MO or standard MO, 20 ng). Bottom: Immunoblotting results and quantification of cytosolic β-catenin levels. For immunoblotting, membrane fraction in embryo lysate was removed using digitonin buffer. Quantitative data are derived from three experiments. (B) hwa fails to induce the body axis in embryos depleted of β-catenin. Embryos were first injected with 20 ng of β-cat-MO or standard MO at one-cell stage and then injected with 200 pg of hwa and 200 pg of β-gal mRNAs into one ventral blastomere at four-cell stage, and were observed at stage 26. (C) dkk1 overexpression inhibits secondary axis induction of wnt8 but not hwa. mRNAs were injected into one ventral blastomere of four-cell stage embryos. Left: Categories of embryos at stage 32. Right: Ratios of embryos in each category. (D) hwa overexpression induces the body axis of embryos derived from oocytes depleted of lrp6. Oocytes were injected with 6 ng of lrp6 antisense oligodeoxynucleotide (lrp6-AS) and one blastomere of the resulting embryos at two-cell stage was injected with 20 pg of hwa mRNA. Embryos were observed at stage 28 (left panel). (E) Embryos from the experiment shown in (D) were also collected at stage 10 for RT-PCR analysis of dorsal markers. qRT-PCR results are based on four technical repeats. *P < 0.05, ***P < 0.001. Scale bars, 1 mm. |
|
Hwa binds to and promotes the degradation of Axin. (A and B) Hwa-Flag physically interacts with Myc-Axin2 (A) and Axin1-HA (B) in HEK293T cells. Zebrafish Hwa and mouse Axin1 and Axin2 were used. XAV939 is a tankyrase inhibitor. Note that Axin1 and Axin2 levels are reduced when zebrafish Hwa is expressed. (C) Direct binding of Hwa with AXIN1. His-AXIN1 (N351) protein of human AXIN1 origin was incubated with GST-Hwa(ΔΝ46) protein of zebrafish origin expressed in E. coli. GST-Hwa (indicated by an arrow) is detected by Western blotting in the His-AXIN1 precipitates. (D) Hwa-HA promotes degradation of endogenous AXIN1 in HEK293T cells. Left: Cells transfected with empty vector (EV) or Hwa-HA were treated with cycloheximide (CHX; 100 μg/ml) and harvested for immunoblotting at indicated time points. Right: Dynamic change of AXIN1 protein level (normalized to α-TUBULIN), shown as mean ± SD. (E) Hwa-Flag binds to endogenous TNKS1/2 in HEK293T cells. (F) Hwa-promoted degradation of endogenous AXIN1 in HEK293T cells is prevented by inhibition of tankyrases. Six hours after transfection, cells were treated with DMSO or 1 μM XAV939 for 18 hours. Relative AXIN1 levels (normalized to α-TUBULIN) are indicated. (G) Immunoblotting reveals an increase of endogenous Axin1 and Axin2 proteins in Mhwatsu01sm mutants at 4 and 6 hpf. Relative levels are indicated. (H) Axin1ΔDIX overexpression rescues the body axes of Mhwatsu01sm mutants. Axin1ΔDIX mRNA was injected into one-cell stage mutants or into one blastomere of 16-cell stage mutants. Embryos at 24 hpf were categorized into five classes (left) and the ratio of embryos in each class is shown (right). |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |