- Title
-
Epigenetic Regulators Modulate Muscle Damage in Duchenne Muscular Dystrophy Model
- Authors
- Bajanca, F., Vandel, L.
- Source
- Full text @ PLoS Curr.
|
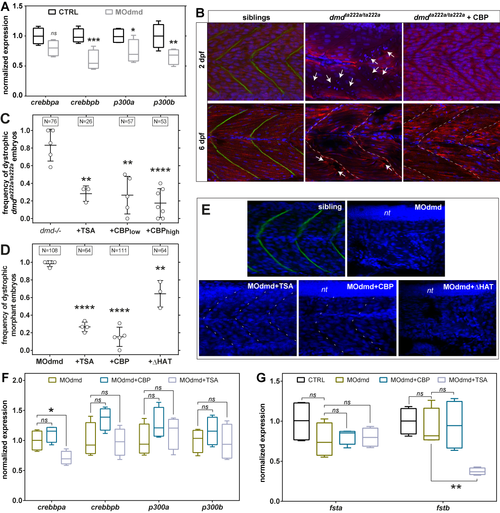
Epigenetic regulators in zebrafish dystrophic embryos. A) Quantitative PCR analysis of p300/CBP transcript expression at 2 days post fertilisation (dpf) in control and morpholino-depleted embryos (MOdmd). (B) Confocal sections of Tg(actc1b:mCherry)pc4 embryos immunostained for Dystrophin (green). Well organised pattern of muscle fibres (mCherry, red) and nuclei (DAPI, blue) in siblings, while heavily disrupted in dmdta222a/ta222a embryos (arrows), showing collapsed and wavy fibres which ends misalign with somatic borders (dashed lines). Overexpressing CBP (dmdta222a/ta222a + CBP; injection of 107 RNA molecules of plasmid per embryo at one cell stage) has a clear positive effect on dystrophic embryos both at 2 and 6 dpf. (C, D) Frequency of dystrophic phenotype (see Materials and Methods) on 2 dpf Dystrophin-null embryos, either dmdta222a/ta222amutants (C) or depleted with MOdmd (D). Overexpressing CBP (low = 10 |
|
(A) Wild-type and MOdmd injected zebrafish embryos at 2 dpf, showing drastic reduction of Dystrophin on embryos injected with morpholino cocktail compared with wild type expression. No secondary developmental defects were observed. (B) Control quantitative PCR with specific primers for crebbp of mouse origin performed on 2 dpf control embryos (non-injected) and embryos injected at one-cell stage with 107 (low), 5×107 (medium) or 108 (high) molecules of mRFP-CBP. Note that: i) endogenous zebrafish crebbp transcripts are not detected in control non-injected embryos; ii) the number of transcripts detected at 2 dpf increases linearly with the increasing number of transcripts injected at one cell stage. (C) Representative confocal sections of 2 dpf wild type embryos injected at one-cell stage with 10-fold doses of RFP-tagged CBP: 107 (low) and 108 (high) RNA molecules per embryo. Embryos were immunostained for exogenous CBP (αRFP; red), Dystrophin (green), and nuclei counterstained with DAPI (blue). Note the distribution of exogenous CBP, accumulated in the nuclei of a small subset of muscle cells when a lower dose is injected (B, red) compared with more widespread expression when a higher dose is injected (C, red). (D) Representative confocal tiled section of a 2 dpf embryo, co-injected at one-cell stage with MOdmd plus 107 RNA molecules (low dose) of RFP-tagged CBP expression plasmid. Panels show immunostaining for Dystrophin (green), exogenous CBP (αRFP; red) and nuclei counterstained with DAPI (blue). Note that despite Dystrophin expression is undetectable the muscles are not damaged, even if only a small subset of total nuclei express exogenous CBP. (E) Control for z-crebbpa and z-crebbpb qPCR primers specificity: neither detects the exogenous CBP of mouse origin. PCR was performed on template pCS2+mRFP-CBP cDNA at concentrations: 100 ng/µl (lines 2-4), 10 ng/µl (lines 5-7) and 1 ng/µl (lines 8-10), and primers: m-crebbp (lines 2, 5, 8), z-crebbpa (lines 3, 6, 9) and z-crebbpb (lines 4, 7, 10). A 100-bp ladder (Biolabs) was used on lines 1 and 11. |