- Title
-
The Macrophage-Specific Promoter mfap4 Allows Live, Long-Term Analysis of Macrophage Behavior during Mycobacterial Infection in Zebrafish
- Authors
- Walton, E.M., Cronan, M.R., Beerman, R.W., Tobin, D.M.
- Source
- Full text @ PLoS One
|
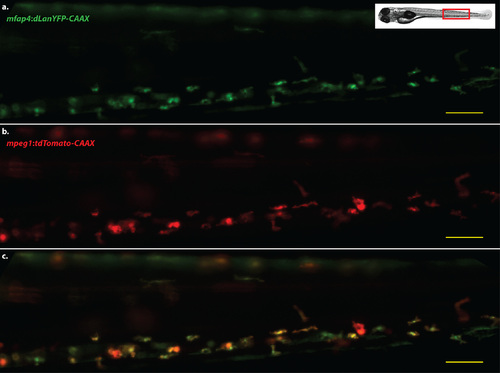
mfap4 transgene expression colocalizes with the expression of mpeg1 transgenes. Representative image of the caudal hematopoietic tissue of a double transgenic larva, four days post-fertilization. a. Cells expressing dLanYFP via the Tg(mfap4:dLanYFP-CAAX)xt11 transgene. b. Cells expressing the tdTomato fluorescent protein via the Tg(mpeg1:tdTomato)xt3 transgene. c. Merged image of a,b. Scale bars = 50 µm. Images representative of at least 40 animals. |
|
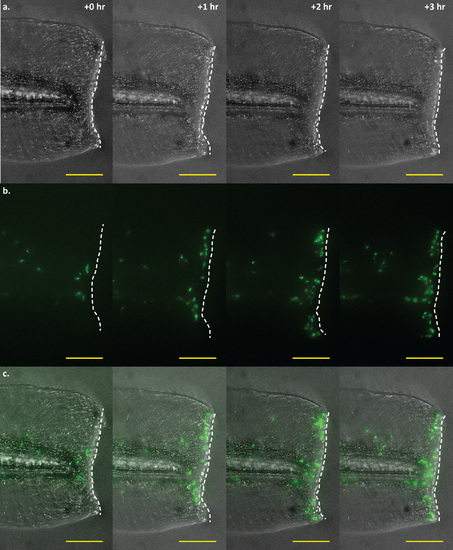
Time-lapse imaging of a tail wound of an mfap4:dLanYFP-CAAX transgenic larva. Time-lapse imaging of macrophage recruitment to the site of a wound at the posterior end of the larval tail. Macrophage movements may be visualized in real-time via fluorescence imaging of mfap4 transgenic larvae. Shown here are four representative frames of such an experiment, showing macrophage recruitment to the wound edge at 0, 1, 2, and 3 hours post-wounding. a. Brightfield detailing the extent and location of the wound. The wound edge is highlighted as a dashed white line. b. Macrophages visualized via dLanYFP fluorescence. The number of cells present proximal to and along the site of the wound can be seen increasing over time as macrophages track toward the damaged tissue. c. Merge of brightfield and fluorescence channels. Scale bars = 100 µm. Images representative of 12 animals from two independent biological replicates. |
|
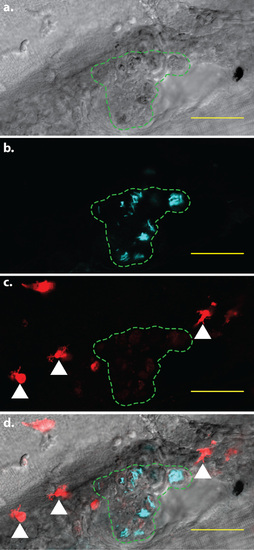
mpeg1-mediated fluorophore expression is attenuated in infected cells. Confocal image of a larval granuloma, 5 days post-infection. The region containing infected cells is indicated by the green dashed line. Within each cell, M. marinum expressing the Cerulean fluorescent protein (cyan signal) are clearly visible. In addition, faint tdTomato fluorescence is visible within the infected cells. White arrowheads indicate examples of bright, uninfected cells exhibiting normal levels of tdTomato fluorescence. a. Brightfield detailing the granuloma in which mpeg1 fluorescent protein expression is attenuated. b. Fluorescent M. marinum in the same area c. Macrophages expressing the tdTomato fluorescent protein. d. Merged image of a-c. Scale bars = 50 µm. |
|
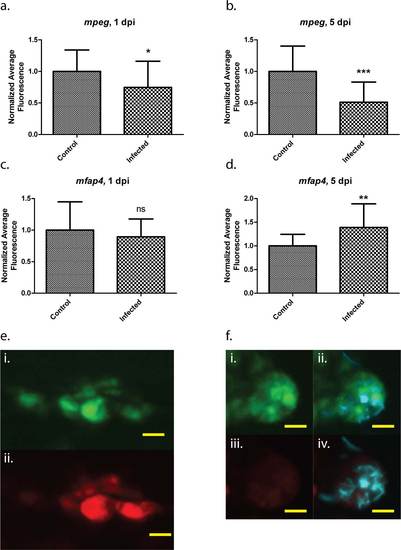
Differential regulation of mfap4 and mpeg1 transgenes during mycobacterial infection. Average fluorescence values of either mpeg1:tdTomato-CAAX or mfap4:dLanYFP-CAAX fluorescent proteins relative to uninfected, age-matched controls. Five randomly chosen infected macrophages per larva (“Infected”), or five randomly chosen macrophages within uninfected larvae (“Control”) were assessed. 20 larvae were analyzed for each of the infected and control groups at 1 dpi as well as for the Infected group at 5 dpi; 19 larvae were analyzed for the Control group at 5 dpi. Both tdTomato fluorescence (a,b) and dLanYFP fluorescence (c,d) were measured for the same pool of cells from each larva. e. Representative image of mfap4 transgene expression (i) and mpeg1 transgene expression (ii) within the same group of cells in an uninfected Control larva, 5 dpi. f. Representative image of mfap4 transgene expression (i) and mpeg1 transgene expression (iii) within the same group of cells in an Infected larva, 5 dpi; also shown are merged images of fluorescent M. marinum (cyan signal) with the YFP (ii) and tdTomato (iv) channels. Note the almost total loss of mpeg1-mediated fluorescence within the infected group of cells, while mfap4 transgene fluorescence remains robust. Scale bars = 10 µm. * p < .05, ** p < .005, *** p = .0002. Student’s t-test with Welch’s correction for unequal variances. Error bars indicate +/- SD. |
|
mfap4:iCre:p2A-tdTomato mediates macrophage-specific gene expression of loxP-containing transgenes. a-d. Zebrafish larva at 3 dpf expressing both Tg(β-actin2:loxP-DsRed-STOP-loxP-EGFP)s928 and Tg(mfap4:iCre:p2A-tdTomato)xt8; a,b. 50x magnification of GFP fluorescence and merge with brightfield, respectively. c,d. 200x magnification of the head, yolk sac, and anterior portion of the gut. e-h. Zebrafish larva at 3 dpf expressing Tg(mfap4:dLanYFP-CAAX)xt11 for comparison. e,f. 50x magnification of dLanYFP fluorescence and merge with brightfield, respectively. g,h. 200x magnification of the head, yolk sac, and anterior portion of the gut. Note that in both larvae, fluorescent protein expression is restricted to macrophages all along the body, with a particular enrichment in the head. Both larvae also exhibit expression within the caudal hematopoietic tissue (CHT). The larvae in a-d show a reduced number of fluorescent cells in the nascent macrophage population that predominates in the CHT region, and modestly reduced fluorescent cell population number in regions where mature macrophages have migrated throughout the body. Scale bars for 50x images = 500 µm; scale bars for 200x images = 100 µm. |
|
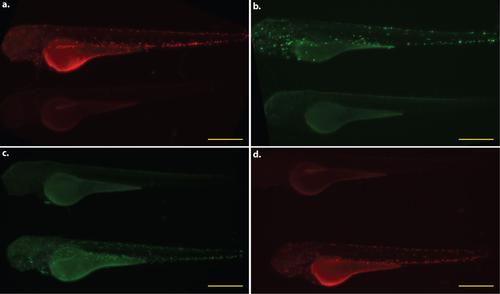
Whole animal expression pattern of mfap4 transgenic lines. All larvae shown are 3 dpf. Each panel pairs a representative transgenic animal with a non-transgenic animal to provide a direct comparison of background fluorescence in the relevant excitation/emission channel. a. Tg(mfap4:tdTomato)xt12. b. Tg(β-actin2:loxP-DsRed-STOP-loxP-EGFP)s928 X Tg(mfap4:iCre:p2A-tdTomato)xt8. c. Tg(mfap4:dLanYFP-CAAX)xt11. d. Tg(mfap4:tdTomato- CAAX)xt6. Scale bars = 500 µm. |
|
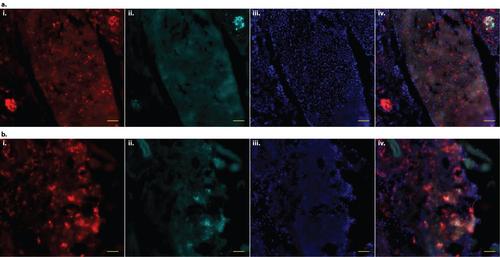
mfap4 transgenes are robustly expressed in adult zebrafish macrophages. a. Frozen 20 µm section of 3 month old adult Tg(mfap4:tdTomato-CAAX)xt6 zebrafish liver tissue, 2 weeks post-infection (wpi) with approximately 400 c.f.u. of M. marinum expressing the Cerulean (cyan) fluorescent protein. i. Macrophages expressing tdTomato. The stellate morphology expected of Kupffer cells, specialized resident macrophages of the liver, can be seen. ii. M. marinum. iii. DAPI. iv. Merge of iiii. b. Frozen 20 µm section of kidney tissue from the same fish. i. Macrophages expressing tdTomato. ii. M. marinum. iii. DAPI. iv. Merge of i-iii. For both a and b, tdTomato (macrophage) and Cerulean (M. marinum) fluorescence signals are derived directly from fixed fluorescent protein; other than DAPI, no additional staining was performed. Scale bars = 50 µm. |