- Title
-
Live imaging of endogenous protein dynamics in zebrafish using chromobodies
- Authors
- Panza, P., Maier, J., Schmees, C., Rothbauer, U., Söllner, C.
- Source
- Full text @ Development
|
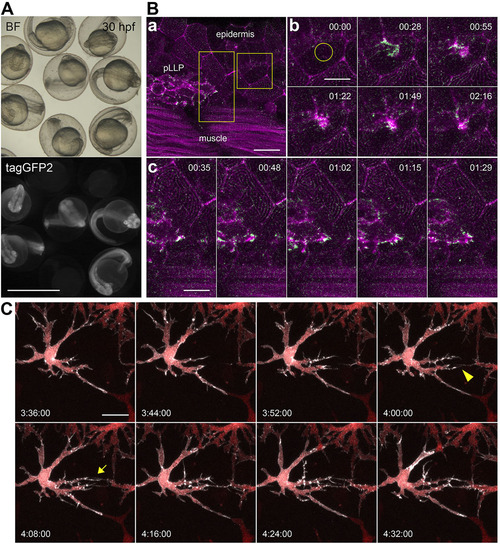
Actin-CB-expressing transgenic zebrafish embryos reveal fast actin dynamics in multiple cell types. (A) 30-hpf embryos from an outcross of hsp70l:Actin-CB founder fish. The embryos were heat-shocked at 24hpf. Chromobody-expressing individuals show widespread fluorescence and no morphological abnormalities when compared with non-transgenic siblings. BF, brightfield. (B) Actin-CB can trace fast protein dynamics in living zebrafish. (a) Overview of a 36hpf embryo, in which actin-CB expression was induced by heat-shock at 24hpf. pLLP cells are visible, together with epidermal cells and muscle fibres. (b) Reorganization of actin after laser-induced wounding (yellow circle). (c) Detailed actin activity at the leading edge of front cells during pLLP migration. Magenta indicates signal from time frame t+1. Green indicates signal obtained from the subtraction of frame t from frame t+1. Green signal highlights the appearance of actin-CB signal from frame to frame (scanning interval is 7s). (C) Filopodial activity during establishment of intercellular contacts between neighbouring xanthophores. Detail of a csf1ra:gal4 embryo expressing UAS:NTR-mcherry (red) and UAS:Actin-CB (white). Filopodia extruded from xanthophore branches are directional to the prospective contact site with neighbouring cells (arrowhead and arrow). Time-stamps: min:s (B), h:min:s (C). Scale bars: 1mm in A; 10µm in Bb,c; 20µm in Ba,C. |
|
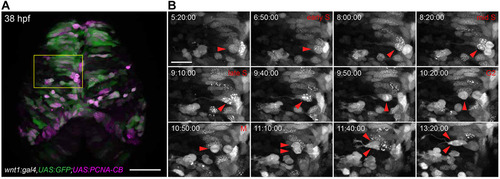
Cell cycle analysis of transgenic zebrafish embryos expressing PCNA-CB. (A) Overview of the dorsal midbrain of a wnt1:gal4,UAS:GFP (green); UAS:PCNA-CB (magenta) double transgenic embryo at 38hpf. (B) Unaltered cell cycle progression in PCNA-CB-expressing, wnt1-positive neural progenitors. PCNA-CB signal transitions from a speckled configuration (S phase) to an evenly distributed one (G2), which precedes mitosis (M). Arrowheads indicate a cell progressing through its last cycle before terminally differentiating into two daughter neurons. Time-stamps: h:min:s. Scale bars: 50µm in A; 20µm in B. |
|
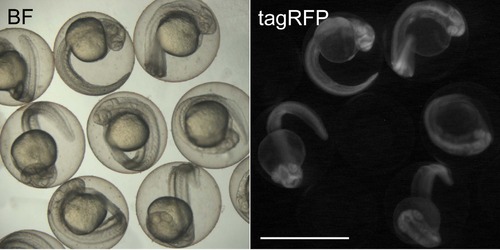
Effect of chromobody induction by heat-shock in 24 hpf hsp70l:PCNA-CB transgenic embryos. 30 hpf embryos heat-shocked at 24 hpf. Ubiquitous fluorescence can be detected after induction. Transgenic embryos appear morphologically similar to non-transgenic siblings, indicating that chromobody expression is well tolerated in vivo. BF: brightfield. Scale bar: 1 mm. |
|
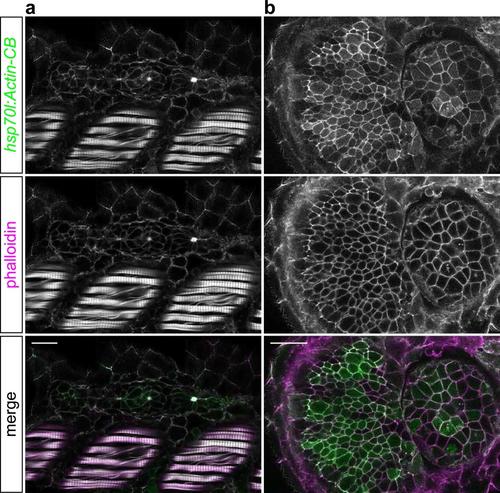
Actin-CB colocalizes with zebrafish F-actin in vivo. 36 hpf hsp70l:Actin-CB embryos heat-shocked at 24 hpf and counterstained with rhodamine phalloidin. (a) Actin-CB fluorescence overlaps F-actin staining in muscle fibres, epidermal cells and in the apically-constricted centres of pLLP rosettes (similar to what has been described in Xu et al., 2014). (b) Actin-CB recognized cortical F-actin in cells of the retina and lens. Scale bars: 20 µm. |
|
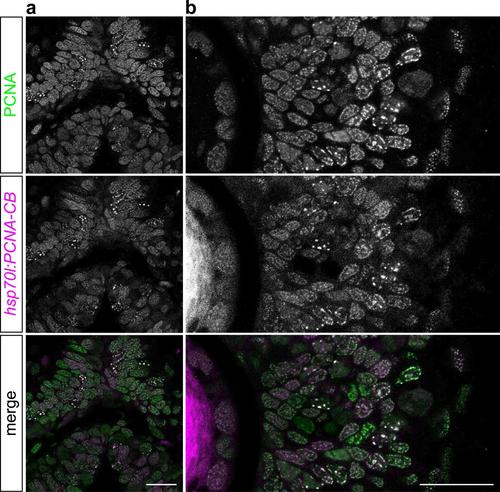
PCNA-CB colocalizes with endogenous zebrafish PCNA in vivo. 36 hpf hsp70l:PCNA-CB embryos heat-shocked at 24 hpf and counterstained using an anti- PCNA antibody. PCNA-CB localizes to nuclear structures that contain endogenous PCNA protein in the dorsal midbrain (a) and retina (b). Scale bars: 20 µm. |
|
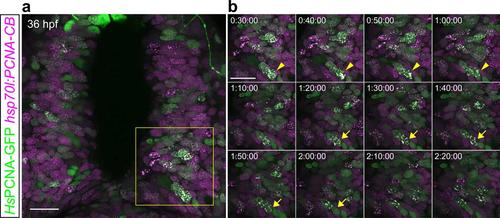
PCNA-CB and human PCNA-GFP dynamically colocalize when expressed in live zebrafish. 36 hpf hsp70l:PCNA-CB embryos transiently expressing human PCNA-GFP from an injected DNA construct. (a) Overview of the scanned area. (b) Detailed time lapse analysis. Live imaging of animals heat-shocked at 24 hpf shows that PCNA-CB and human PCNA-GFP mark the same nuclear structures and their pattern is characteristic of S phase. Additionally, both fluorescent fusions can trace these structures’ dynamics (arrowheads and arrows indicate two different cells over time). Scale bars: 20 µm. Timestamps: h:min:s. |