- Title
-
CLP1 Founder Mutation Links tRNA Splicing and Maturation to Cerebellar Development and Neurodegeneration
- Authors
- Schaffer, A.E., Eggens, V.R., Caglayan, A.O., Reuter, M.S., Scott, E., Coufal, N.G., Silhavy, J.L., Xue, Y., Kayserili, H., Yasuno, K., Rosti, R.O., Abdellateef, M., Caglar, C., Kasher, P.R., Cazemier, J.L., Weterman, M.A., Cantagrel, V., Cai, N., Zweier, C., Altunoglu, U., Satkin, N.B., Aktar, F., Tuysuz, B., Yalcinkaya, C., Caksen, H., Bilguvar, K., Fu, X.D., Trotta, C.R., Gabriel, S., Reis, A., Gunel, M., Baas, F., Gleeson, J.G.
- Source
- Full text @ Cell
|
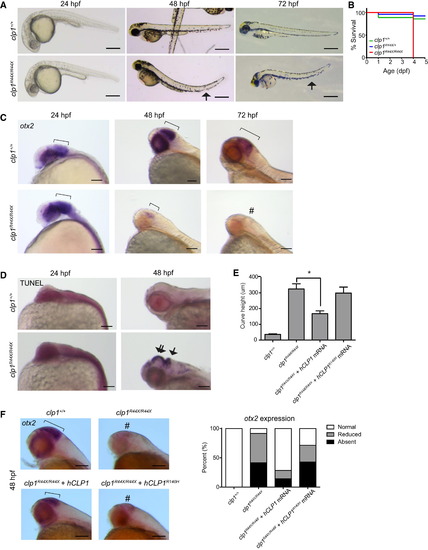
Zebrafish clp1R44X Homozygous Mutants Show Gross Brain Defects, Reduced Survival, and Neurodegeneration (A) Gross morphology of WT and clp1R44X/R44X zebrafish mutants showed misshapen head, small eyes, and curved tail (arrow), suggesting neuromotor defects. Scale bar, 500 µm. (B) Kaplan-Meier curve showed reduced survival of clp1R44X/R44X fish (additional allele shown in Figure S3. (C) Progressively reduced otx2 expression in developing clp1R44X/R44X zebrafish brains. Broad otx2 expression domain at 24 hpf was unremarkable in mutant (bracket), suggesting that initial patterning was not disrupted. From 48–72 hpf, WT fish showed expression restricted to midbrain-hindbrain organizer (bracket), whereas mutant showed weak expression, completely absent by 72 hpf (#). (D) TUNEL-positive cells were increased in mutant at 48 hpf in both the hindbrain (arrow) and the midbrain/diencephalon (double arrow), further investigated in Figure S3. (E) Partial rescue of the clp1R44X/R44X phenotype with human WT, but not p.R140H CLP1, mRNA by measuring curved tail height. No difference was detected between uninjected and injected with p.R140H mRNA, whereas WT mRNA partially recovered curved tail phenotype. p <0.01, Student’s t test. Error bar, SEM. (F) In situ for otx2 in WT, clp1R44X/R44X, and clp1R44X/R44X injected with human CLP1 mRNA. Human CLP1, but not CLP1R140H, prevented the loss of otx2 expression at 48 hpf in clp1 mutants, quantified at right. n >25 embryos each condition. Scale bar, 100 µm. EXPRESSION / LABELING:
|
|
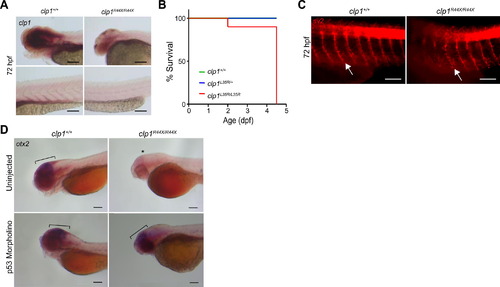
clp1 Mutant Zebrafish Showed Reduced Lifespan, Motor Neuron Fragmentation, and p53-Mediated Neural Loss, Related to Figure 3 (A) In situ hybridization showed clp1 expressed in developing brain and motor neurons of clp1+/+ zebrafish at 72 hours postfertilization (hpf) but not clp1R44X/R44X. (B) Lifespan of clp1L35R/L35R mutant zebrafish is reduced to 4.5 days compared to heterozygous or WT clutchmates. (C) Sv2 immunostaining showed motor neuron fragmentation in clp1R44X/R44X zebrafish compared to control clutchmates at 72 hpf, concordant with neural degeneration. (D) In situ hybridization at 72 hpf for otx2 in clp1+/+ and clp1R44R/R44X zebrafish injected with tp53 antisense morpholino, or uninjected, showed tp53 knockdown restores otx2 expression in mutant zebrafish to near WT levels. Black scale bar, 100 µm, white scale bar, 50 µm. EXPRESSION / LABELING:
PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|
Reprinted from Cell, 157, Schaffer, A.E., Eggens, V.R., Caglayan, A.O., Reuter, M.S., Scott, E., Coufal, N.G., Silhavy, J.L., Xue, Y., Kayserili, H., Yasuno, K., Rosti, R.O., Abdellateef, M., Caglar, C., Kasher, P.R., Cazemier, J.L., Weterman, M.A., Cantagrel, V., Cai, N., Zweier, C., Altunoglu, U., Satkin, N.B., Aktar, F., Tuysuz, B., Yalcinkaya, C., Caksen, H., Bilguvar, K., Fu, X.D., Trotta, C.R., Gabriel, S., Reis, A., Gunel, M., Baas, F., Gleeson, J.G., CLP1 Founder Mutation Links tRNA Splicing and Maturation to Cerebellar Development and Neurodegeneration, 651-63, Copyright (2014) with permission from Elsevier. Full text @ Cell