- Title
-
Swap70b is required for convergent and extension cell movement during zebrafish gastrulation linking Wnt11 signalling and RhoA effector function
- Authors
- Xu, X., Shuen, W.H., Chen, C., Goudevenou, K., Jones, P., and Sablitzky, F.
- Source
- Full text @ Dev. Biol.
|
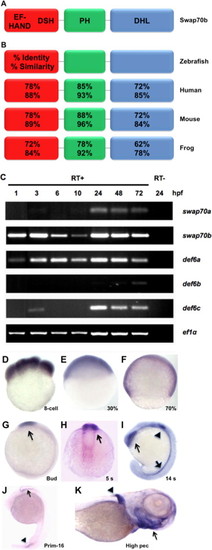
Swap70b expression during zebrafish development. (A) Schematic representation of zebrafish Swap70b with putative Ca2+-binding EF-hand motifs at the N-terminus followed by a Def6/Swap70 homology (DSH) domain, a pleckstrin-homology (PH) domain at the centre, and a Dbl-homology-like (DHL) domain that contains a coiled coil motif at the C-terminus. (B) The numbers refer to amino acid identities and similarities between the different orthologues. (C) Expression profiles of swap70/def6 paralogues during zebrafish development. RT-PCR was performed to indicate the expression of five swap70/def6 paralogues at various developmental stages. hpf: hours post-fertilisation. PCR reactions without reverse transcription (RT-) are shown for 24 hpf only and ef1α expression served as an internal control. (D–J) In situ hybridisation with a swap70b-specific antisense probe at different developmental stages: (D) 8-cell; (E) 30% epiboly (30%); (F) 70% epiboly (70%); (G) bud; (H) 5 somites (5 s); (I) 14 somites (14 s); (J) primordium 16 (prim-16) and (K) high pectoral (high pec). Sense control probes did not give rise to any signal (data not shown). EXPRESSION / LABELING:
|
|
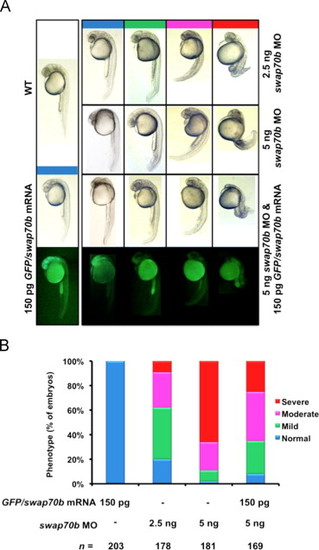
Swap70 morphants exhibiting reduced length of the body axis can be rescued by ectopic expression of GFP/Swap70b fusion protein. (A) Zebrafish embryos were either un-injected (WT) or injected with GFP/swap70b mRNA (150 pg), swap70b MO1 (2.5 ng, 5 ng) or co-injected with GFP/swap70b mRNA (150 pg) and swap70b MO1 (5 ng). Swap70 morphants at 24 hpf were grouped according to the severity of their morphological phenotypes into normal (blue), mild (green), moderate (pink) and severe (red). Images of representative embryos are shown. (B) Quantification of the phenotypic analysis. n: total number of embryos derived from at least three independent experiments. PHENOTYPE:
|
|
Swap70b morphants exhibit a shorter body axis. Zebrafish embryos either un-injected (WT; A) or injected with GFP/swap70b mRNA (150 pg) (B), with swap70b MO1 (5 ng) alone (C), or co-injected with GFP/swap70b mRNA (150 pg) and swap70b MO1 (5 ng) (D) are shown at 10 1/3 hpf. (E) To assess the length of the body axis the angle between anterior and posterior ends was measured in wild type (WT; red arrows) and compared to the angle in morphant embryos (blue arrows). Standard deviation (error bars) is shown. Two-tailed Student′s t-test was performed to establish significance. ***: P<0.001. N.S.: not significantly different. PHENOTYPE:
|
|
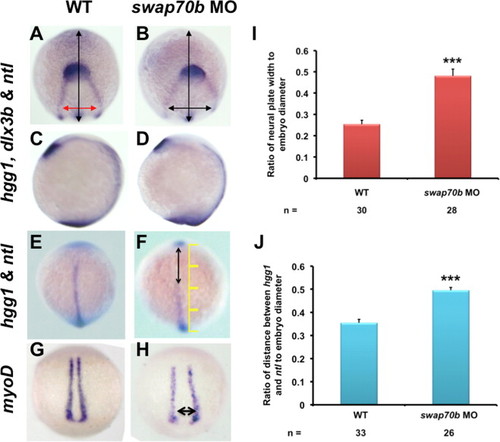
CE cell movements are impaired in swap70b morphants. In situ hybridisation of un-injected embryos (A, C, E, G) and embryos injected with 5 ng swap70b MO1 (B, D, F, H) were performed at tail-bud stage with hgg1 (marker of the polster/prechordal plate), ntl (marker of the notochord) and dlx3b (marker of the edges of the neural plate). Medio-lateral narrowing (convergence) was quantified by measuring the ratio (I) of width of the dlx3b staining (red/black double-headed arrow) at a constant distance (1/4 of the embryo diameter) from the dlx3b arc to the embryo width (vertical black arrow). The extent of anterior–posterior lengthening (extension) was quantified by measuring the ratio (J) of the distance between hgg1 and ntl (double-headed arrow) to the embryo width (yellow scale). Two-tailed Student′s t-tests were performed to determine statistical significance. ***: P<0.001. In situ hybridisation with a myoD (G, H) revealed widening of the body axis (double-headed arrow) in Swab70b morphants. PHENOTYPE:
|
|
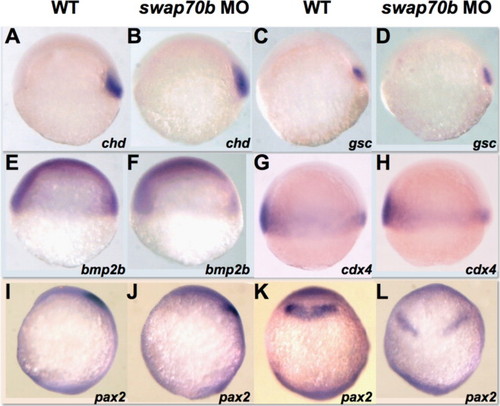
Cell fate specification appears normal in swap70b morphants. Un-injected and 5 ng swap70b MO1-injected embryos at 6 hpf were hybridised with various probes: Chordin, chd (A, B); goosecoid, gsc (C, D); bone morphogenetic protein 2b, bmp2b (E, F); caudal homeobox transcription factor 4, cdx4 (G, H). At tail-bud stage, the expression pattern of an anterior specific gene pax2 shifted posteriorly and became broader in Swap70b morphants, but was still in the midbrain-hindbrain boundary (I–L). Lateral views (A–J) and dorsal views (K, L) with anterior to the top are shown. PHENOTYPE:
|
|
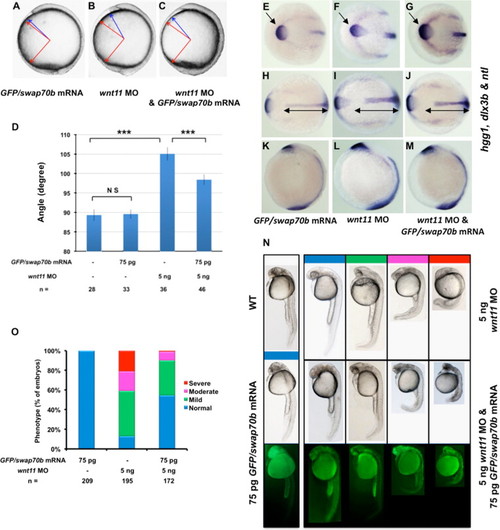
Swap70b acts downstream of Wnt11. Zebrafish embryos at 2-cell stage were injected with GFP/swap70b mRNA (75 pg) (A), wnt11 MO (5 ng) alone (B), or co-injected with GFP/swap70b mRNA (75 pg) and wnt11 MO (5 ng) (C). (D) Quantification of A-P axis extension at 10 1/3 hpf. The angle between the most anterior and posterior embryonic structure was measured and quantified as described in Fig. 3. Two-tailed Student′s t-tests were used to determine statistical significance. ***: P<0.001. (E–M) In situ hybridisation with hgg1, dlx3b and ntl as indicated. Black double-headed arrows in H–J are given as reference to the WT. Arrows mark the hgg1 expression domain indicating the altered position and shape of the polster/prechordal plate in Wnt11 morphants (F) that is essentially rescued by ectopic expression of GFP/Swap70b (G). (N) At 24 hpf stage, embryos were grouped into normal (blue), mild (green), moderate (pink) and severe (red) according to their morphological phenotype. Representative images of embryos are shown. Each panel of the bottom two rows depict identical embryos. Green fluorescence in yolk and yolk extension is due to auto-fluorescence. (O) Bar graphs show the percentage of embryos with normal, mild, moderate and severe phenotypes. n: total number of embryos derived from at least three independent experiments. PHENOTYPE:
|
|
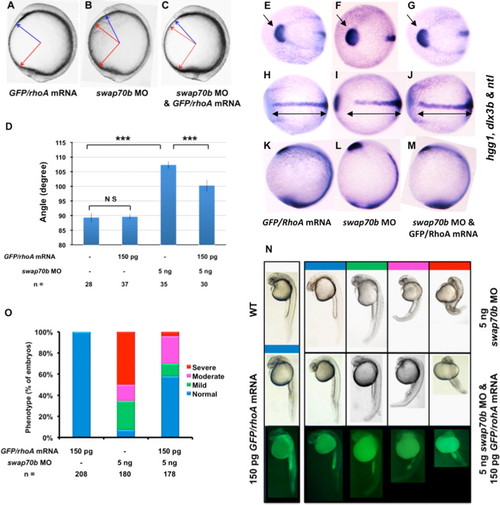
Swap70b modulates CE cell movements via RhoA. Zebrafish embryos at 2-cell stage were injected with GFP/rhoA mRNA (150 pg) (A), swap70b MO (5 ng) alone (B), or co-injected with GFP/rhoA mRNA (150 pg) and swap70b (5 ng) (C). (D) Quantification of A-P axis extension at 10 1/3 hpf. The angle between the most anterior and posterior embryonic structure was measured and quantified as described in Fig. 3. Two-tailed Student′s t-tests were used to determine statistical significance. ***: P<0.001. (E–M) In situ hybridisation with hgg1, dlx3b and ntl as indicated. Black double-headed arrows in H–J are given as reference to the WT. Arrows mark the hgg1 expression domain indicating the altered position and shape of the polster/prechordal plate in Swap70b morphants (F) that is essentially rescued by ectopic expression of GFP/RhoA (G). (N) At 24 hpf stage, embryos were grouped into normal (blue), mild (green), moderate (pink) and severe (red) according to their morphological phenotype. Representative images of embryos are shown. (O) Bar graphs show the percentage of embryos with normal, mild, moderate and severe phenotypes. n: total number of embryos derived from at least three independent experiments. |
|
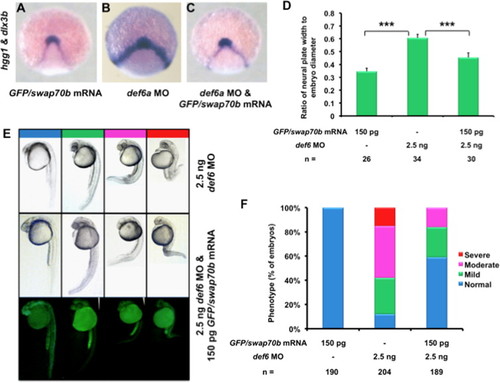
Ectopic GFP/Swap70b expression rescued Def6a morphants. Embryos at 2-cell stage were injected with GFP/swap70b mRNA (150 pg) (A), def6a MO (2.5 ng) alone (B), or GFP/swap70b mRNA (150 pg) and def6a MO (2.5 ng) (C) and were stained at tail-bud stage with hgg1 and dlx3b to evaluate CE using the same quantification method described above ( Fig. 4I). (D) Two-tailed Student′s t-tests were used to determine statistical significance. ***: P<0.001. (E) Later at 24 hpf, embryos were grouped into normal (blue), mild (green), moderate (pink) and severe (red) according to their morphological phenotype. Representative images of embryos are shown. (F) Bar graphs show the percentage of embryos with normal, mild, moderate and severe phenotypes. Only 1/189 of the rescued embryos exhibited a severe phenotype as shown in E. n: total number of embryos derived from at least three independent experiments. PHENOTYPE:
|
|
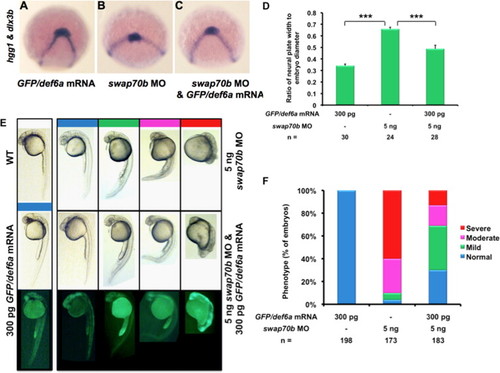
Ectopic GFP/Def6a expression rescued Swap70b morphants. Embryos at 2-cell stage were injected with GFP/def6a mRNA (300 pg) (A), swap70b MO (5 ng) alone (B), or GFP/def6a mRNA (300 pg) and swap70b MO (5 ng) (C) and were stained at tail-bud stage with hgg1 and dlx3b to evaluate CE using the same quantification method described above ( Fig. 4I). (D) Two-tailed Student′s t-tests were used to determine statistical significance. ***: P<0.001. (E) Embryos at 24 hpf were grouped into normal (blue), mild (green), moderate (pink) and severe (red) according to their morphological phenotype. Representative images of embryos are shown. (F) Bar graphs show the percentage of embryos with normal, mild, moderate and severe phenotypes. n: total number of embryos derived from at least three independent experiments. |
|
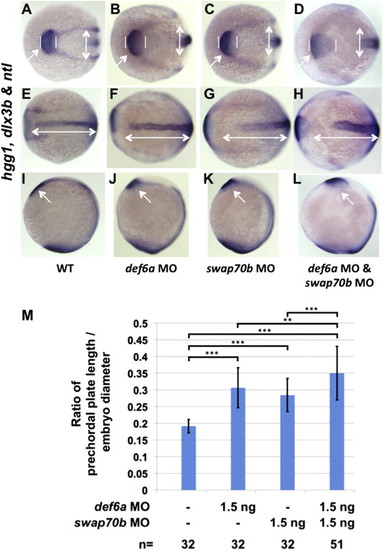
Def6a and Swap70b have distinct functions in CE cell movement. In situ hybridisation with hgg1, dlx3b and ntl as indicated comparing WT embryos (A, E, I) with either Def6a (B, F, J), Swap70b (C, G, K) single morphants or Def6a/Swap70b double mutants (D, H, L). White double-headed arrows in E–H are given as reference to the WT. Arrows and vertical lines mark the hgg1 expression domain indicating the altered position and shape of the polster/prechordal plate in Def6a (B, J), Swap70b (C, K) and Def6a/Swap70b (D, L) morphants compared to WT (A, I). (M) Quantification of the altered shape of the polster/prechordal plate. The ratio of prechordal plate length to embryo diameter is shown for wild type embryos (0.19±0.02), Def6a morphants (0.31±0.06), Swap70b morphants (0.29±0.05) and double mutant morphants (0.35±0.08). Two-tailed Student′s t-tests were used to determine statistical significance. ***: P<0.001. **: P<0.01. Number of embryos (n) in each group analysed as indicated. PHENOTYPE:
|
|
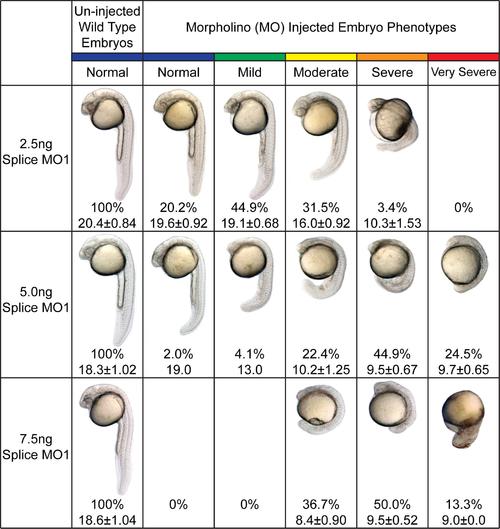
Phenotypes of splice MO1-mediated Swap70b knockdown. Embryos injected with 2.5 ng, 5.0 ng or 7.5 ng of splice MO1 were analysed at 24 hpf and grouped according to their morphological phenotypes into normal (blue), mild (green), moderate (yellow), severe (orange), and very severe (red). Un-injected wild type embryos served as experimental control. The number of surviving embryos in each group is shown in percentages. The lengths (mm) of embryos were measured from head to tail (using 10 X magnification) and are presented as mean ± standard derivation or just the mean in case of less than 3 embryos found in the group. |

Phenotypes of splice MO2-mediated Swap70b knockdown. Embryos injected with 2.5 ng, 5.0 ng, 7.5 ng or 10 ng of splice MO2 were analysed at 24 hpf and grouped according to their morphological phenotypes into normal (blue), mild (green), moderate (yellow), severe (orange), and very severe (red). Un-injected wild type embryos served as experimental control. The number of surviving embryos in each group is shown in percentages. The lengths (mm) of embryos were measured from head to tail (using 10 X magnification) and are presented as mean ± standard derivation or just the mean in case of less than 3 embryos found in the group. |

Wnt5b/Swap70b double mutant embryos exhibit enhanced CE cell movement phenotype. In situ hybridisation with hgg1, dlx3b and ntl as indicated comparing WT embryos with either Wnt5b or Swap70b single morphants and Wnt5b/Swap70b double mutants as indicated. White double-headed arrows shown are are given as reference to the WT. |
Reprinted from Developmental Biology, 386(1), Xu, X., Shuen, W.H., Chen, C., Goudevenou, K., Jones, P., and Sablitzky, F., Swap70b is required for convergent and extension cell movement during zebrafish gastrulation linking Wnt11 signalling and RhoA effector function, 191-203, Copyright (2014) with permission from Elsevier. Full text @ Dev. Biol.