- Title
-
Complex cardiac defects after ethanol exposure during discrete cardiogenic events in zebrafish: Prevention with folic acid
- Authors
- Sarmah, S., and Marrs, J.A.
- Source
- Full text @ Dev. Dyn.
|
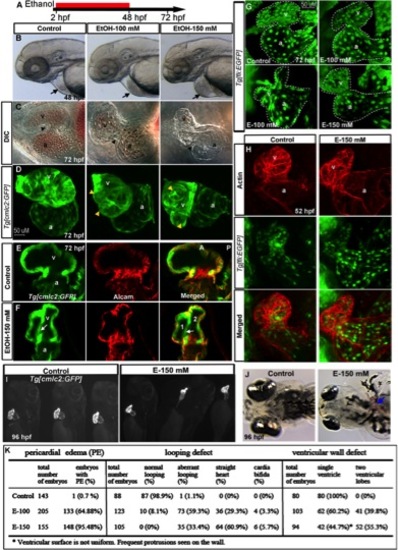
Chronic ethanol exposure during cardiogenesis severely disrupts heart formation. A: Schematic diagram showing the developmental timing (2–48 hpf) of ethanol exposure. B, C: Live images showed pericardial edema (arrows) (B), and smaller, misshapen chambers with aberrant AV canal (arrowheads) in the ethanol-treated embryos (C). D, G: 3D renderings of confocal sections of Tg[cmlc2:GFP] and Tg[fli1:EGFP] embryos showing distorted myocardium, multilobed ventricle (D), and misshapen endocardium (G) in ethanol-exposed embryos. Yellow arrowheads, multiple lobes. E, F: Single confocal sections at the AV boundary of alcam-stained Tg[cmlc2:GFP] embryos showing a single ventricle in the control and two ventricular lobes (1 and 2) in the ethanol-treated embryo. White arrows, abnormal additional ventricular wall connection. H: 3D Confocal images of Texas red phalloidin–stained Tg[fli1:EGFP] embryos showing defective endocardium corresponding to the myocardium in ethanol exposed embryos. I, J: Brightfield live images of Tg[cmlc2:GFP] embryos showed abnormal heart (I) and blood clot (J; blue arrow) at 96 hpf in ethanol-exposed embryos. K: Scores showing the effect of ethanol on cardiac development (results from three individual experiments). Mantel-Haenszel chi-square tests: PE, P < 0.0001; looping defect, P < 0.0001; ventricular wall defect, P < 0.0001. a, atrium; v, ventricle. Anterior to the left (B–H, J), anterior to the top (I). |
|
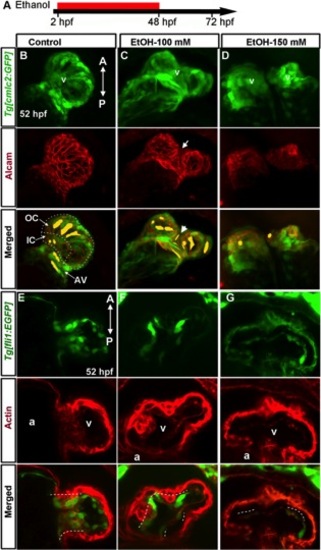
Chronic ethanol exposure during cardiogenesis produces defective chambers and endocardial cushion. A: Schematic diagram showing the developmental timing (2–48 hpf) of ethanol exposure. B–D: Ethanol-treated embryos do not have stereotypical regionally confined cardiomyocyte shapes. Extended focus images of confocal sections from control Tg[cmlc2:GFP] embryo (B) costained with alcam antibody showed large, elongated cardiomyocytes in the outer curvature (OC) and AV region, and small rounded cardiomyocyte in the inner curvature (IC) of the ventriclular surface in control embryos; the orientation of the cells was aligned with each other. C: Cardiomyocytes were disorganized, showing variable sizes and shapes in ethanol treated embryos. D: Ethanol treatment occasionally caused cardia bifida, which display small cardiomyocytes. Representative cell shapes are highlighted in yellow; white arrow, defective ventricular wall; white arrowhead, abnormal thin cell. E–G: Severely defective endocardial cushion formation was seen after ethanol treatment. E: Confocal sections of the control 52-hpf Tg[fli1:EGFP] embryo heart co-labeled with Texas red-phalloidin (F-actin) showed clusters of GFP- and F-actin-positive cells (white dotted line) at the AV boundary, which were reduced (F) or absent (G) in ethanol treated embryos. A, anterior; P, posterior; a, atrium; v, ventricle. |
|
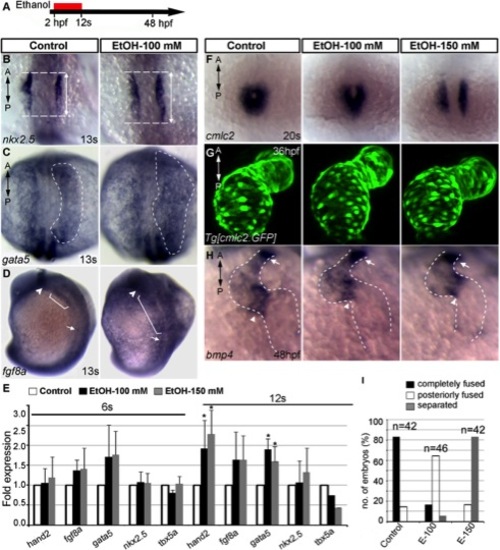
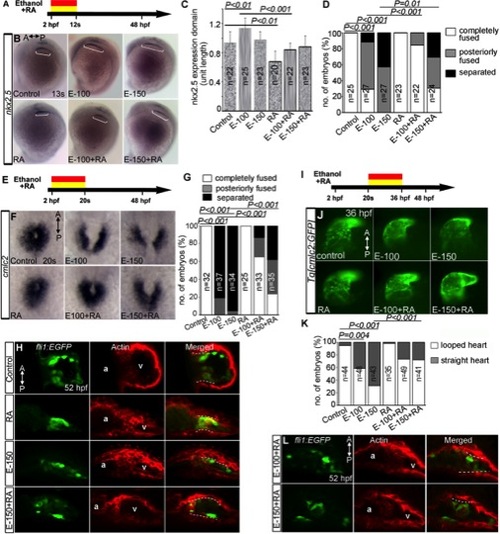
Ethanol exposure during gastrulation through cardiac specification induces defects in cardiac gene expression and myocardial migration. A: Schematic diagram shows the timing of ethanol exposure. B–D: Whole mount ISH at 13s stages showed moderate expansion of expression domains for nkx2.5 (B) (see Fig. 7C for statistical significance), gata5 (C), and fgf8a (D) in the ethanol-treated embryos, as compared to control. E: Transcript levels of genes expressed in differentiating myocardial cells increased after ethanol treatment. Quantitative RT-PCR assays comparing cardiogenic gene expression levels at 6s and 12s stages (average fold change from at least 3 independent experiments), Student′s t-test: *P<0.01. F: ISH detecting cmlc2 at 20s stage showed cardiac fusion delay after ethanol treatment (2 hpf-12s). G: Live images of Tg[cmlc2:GFP] embryos exposed to ethanol (2 hpf-12s) showed normal cardiac looping and normal chamber morphology. H: bmp4 expression examined by ISH. Ethanol-exposed (2 hpf-12s) embryos showed normal bmp4 expression pattern. Arrowheads, AV boundary; arrows, OFT; dotted line, cardiac silhouette. I: Graph illustrates myocardial fusion differences at 20s stage in control and ethanol-treated embryos. Mantel-Haenszel chi-square tests: P<0.0001 for both control versus EtOH-100 and control versus EtOH-150. n, number of embryos; A, anterior; P, posterior. |
|
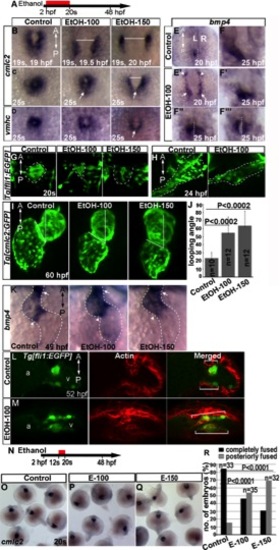
Ethanol exposure until cardiac fusion produces defects that persist at later stages of development. A, N: Schematic diagrams showing the timing of ethanol exposure. B–D: ISH of cmlc2 expression at 19s and 25s; vmhc expression at 25s showed delay in myocardial fusion in ethanol-exposed embryos. Arrows, posterior fusion. E, E2: bmp4 expression at 20 hpf. E: Control. E2: Ethanol-exposed embryos. Arrow, cardiac primordium; arrowhead: posterior retina. F–F32: bmp4 expression at 25 hpf. F: Control. F2–F23: representatives of ethanol-exposed embryos. White dotted line represents midline of the body. G, H: Tg[fli1:EGFP] embryo images at 20s (G) and 24 hpf (H) stages showed defective endocardium formation after ethanol exposure. I: Tg[cmlc2:GFP] embryo images showed aberrant looping angle. J: Graph shows quantification of looping angle. K: bmp4 expression in the control and ethanol-exposed embryos at 48 hpf. Arrowhead, AV boundary; arrow, OFT. L, M: Stained Tg[fli1:EGFP] embryos showed dispersion of GFP and F-actin-positive cells (white brackets) throughout the ventricle after ethanol exposure. N–R: Ethanol exposure only during myocardial migration (12s–20s) causes modest fusion delay. O–Q: ISH showed cmlc2 expression. O: Control. P,Q: ethanol-exposed embryos. R: Graph shows quantification of myocardial precursors fusion. P value: Mantel-Haenszel chi-square tests. A, anterior; P, posterior. |
|
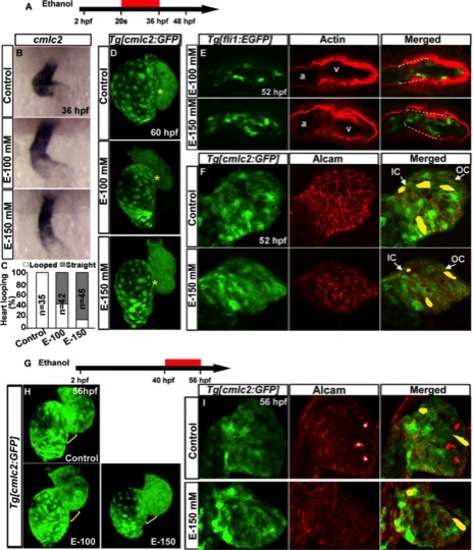
Ethanol exposure during cardiac looping caused abnormal heart morphogenesis and defective endocardial cushions. A, G: Schematic diagram showing ethanol exposure timing. B: ISH detecting cmlc2 at 36 hpf: control, normal looping; EtOH-100 mM, modest looping; EtOH-150 mM, straight heart tube. C: Graph quantifies looped and straight heart at 36 hpf. D: 3D confocal images of Tg[cmlc2:GFP] embryos: control, normal heart chambers; ethanol-treated embryos, aberrant heart chambers. Yellow asterisks, AV canal. E: Ethanol-treated Tg[fli1:EGFP] embryos showed dispersed GFP and F-actin-positive cells (white dashed line) throughout the ventricle (compare control panel 2E). F: Alcam-stained Tg[cmlc2:GFP] embryo showed similar distribution of cardiomyocytes in the control and ethanol-treated embryos. G–I: Ethanol exposure during cardiac chamber growth did not lead to defective chamber wall formation. H: 3D confocal images of Tg[cmlc2:GFP] embryos. Control and E-100 mM, normal heart; E-150, nearly normal heart chamber. Yellow brackets, AV region. I: Alcam-stained Tg[cmlc2:GFP] embryos showed similar pattern of cardiomyocyte distribution in the ventricular wall in ethanol-treated and control embryos. White asterisk, alcam-positive hatching gland cells, which are outside the heart but seen in this 3D rendering. Representative cell shapes are highlighted in yellow. Anterior to the top. |
|
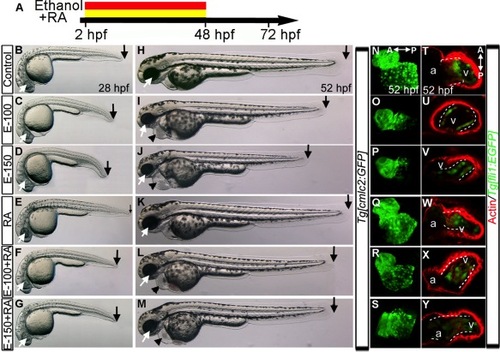
RA Supplementation during chronic ethanol treatment (2–48hpf) does not restore normal heart development. A: Schematic diagram showing the timing of ethanol and RA exposure. B–M: Live images of embryos at 28 (B–G) and 52 hpf (H–M) showed normal morphology in the control (B, H) and RA-treated (E, K) embryos. Ethanol-treated embryos exhibited small eye, shorter body (C, D, I, J) and pericardial edema (I, J). Ethanol+RA-treated embryos showed near normal eye size, slightly shorter body (F, G, L, M) and pericardial edema (L, M). Arrows, black, posterior end of the embryos; white arrow, eye, black arrowhead, pericardial edema. N–S: 3D renderings of fixed Tg[cmlc2:GFP] embryo confocal sections showed normal cardiac morphology in control (N) and RA-treated embryos (Q); straight hearts in ethanol-treated embryos (O, P); near normal or straight heart in ethanol+RA-cotreated (R, S) embryos. T–Y: Phalloidin-stained Tg[fli1:EGFP] embryos: clustering of GFP/F-actin-positive cells (white dashed line) at the AV boundary in the control (T) and RA-treated embryos (W); reduction and dispersion of GFP/ F-actin positive cells in ethanol-treated embryos (U, V); dispersion of GFP/F-actin-positive cells throughout the ventricle in ethanol+RA cotreated embryos (X, Y). A, anterior; P, posterior. |
|
RA supplementation during acute ethanol treatment partially rescues a subset of cardiac development defect. A, E, I: Schematic diagrams showing the timing of ethanol and RA exposure. B: ISH showed nkx2.5 expression (white brackets) in the control, ethanol, RA, and ethanol+RA treated embryos (2hpf–12s). C: Graph shows the quantification of nkx2.5 expressing domain length after ethanol exposure (2hpf–12s). D: Graph shows quantification of myocardial fusion at 20s after ethanol exposure (2hpf–12s). F: Expression of cmlc2 in the control, ethanol, RA, and ethanol+RA treated embryos (2hpf–20s). G: Graph shows the quantification of myocardial fusion. H: Stained Tg[fli1:EGFP] embryos showed the dispersion of GFP and F-actin-positive cells (white dashed line) throughout the ventricle in the ethanol and ethanol+RA cotreated embryos (2hpf–20s). J: Brightfield images of Tg[cmlc2:GFP] embryos showed hearts of control, ethanol, RA, and ethanol+RA treated (20s–36hpf) embryos. K: Graph showing the quantification of looped and straight heart (as in E-150) at 36 hpf (L) Stained Tg[fli1:EGFP] embryos showed reduced clustering of GFP, F-actin double positive cells near the AV boundary in ethanol+RA treated (20s–36 hpf) embryos (compare control panel 7H; ethanol 5E). Statistical analyses: C, Student′s t-tests; D, G, K, Mantel-Haenszel chi-square tests. |
|
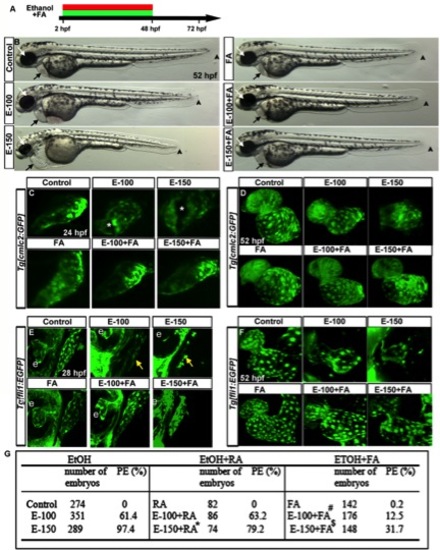
FA supplementation during chronic ethanol exposure (2–48hpf) rescues ethanol-induced cardiac defects. A: Schematic diagram showing the timing of ethanol and FA exposure. B: Live images showed heart edema, small eye, and short body length in the ethanol-exposed embryos and normal morphology in FA cosupplemented (2–48hpf) embryos. Black arrow, heart; white arrow, eye; arrowhead, posterior end of the embryo. C, D: Confocal images of Tg[cmlc2:GFP] embryos showed linear heart tube (24 hpf) and normal chambers (52 hpf) in the control, FA-treated, and ethanol+FA-cotreated embryos. Ethanol-exposed embryos exhibited abnormal heart tube with defective myocardial fusion (asterisk, 24 hpf) and misshapen hearts (52 hpf). E, F: Confocal images of Tg[fli1:EGFP] at 28 and 52 hpf showed misshapen endocardium (yellow arrows; e, eye) in the ethanol-treated; normal endocardium in control, FA-treated, and E-100+FA-cotreated; and near normal endocardium in E-150+FA-cotreated embryos. G: Scores showing the effect of continuous supplement of RA or FA with ethanol on pericardial edema (PE). Results shown were calculated from three individual experiments. (Mantel-Haenszel chi-square tests: control vs. EtOH-100 or EtOH-150, P < 0.0001; control vs. RA, P = 1.00; EtOH-150 vs. EtOH-150+RA, P < 0.0001 (*); control vs. FA, P = 0.01; EtOH-100 vs. EtOH-100+FA, P < 0.0001 (#); EtOH-150 vs. EtOH-150+FA ml, P < 0.0001 ($)). |
|
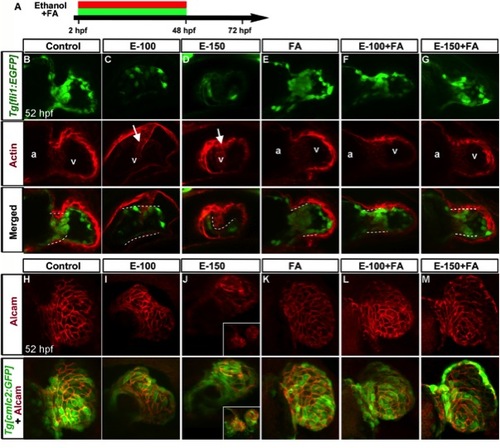
Folic acid supplementation during chronic ethanol exposure (2–48 hpf) rescues ethanol-induced endocardial cushion and chamber formation defects. A: Schematic diagram showing the timing of ethanol and FA exposure. B–G: Endocardial cushion formation was restored in ethanol-exposed, FA co-supplemented embryos. Stained Tg[fli1:EGFP] embryos showed clustering of GFP- and F-actin-positive cells (white dashed line) in the control, FA-treated, and ethanol+FA-cotreated embryos. Ethanol-treated embryos exhibited no clustering of GFP- and F-actin-positive cells at the AV boundary. White arrow, defective wall in the ventricle. a, atrium; v, ventricle. Atrium in the ethanol-treated embryos cannot be seen in this view. H–M: Ethanol-induced ventricular chamber defects were rescued in the FA-cosupplemented embryos. Alcam-stained Tg[cmlc2:GFP] embryos showed a similar pattern of cardiomyocyte distribution in the ventricular wall of control, FA-treated, and ethanol+FA-cotreated embryos; cardiomyocyte sizes and shapes were variable throughout the ventricular wall in the ethanol-treated embryos. Inset (J) in 150 mM ethanol showed cardia bifida. Anterior to the top. |