- Title
-
Antagonism and synergy between extracellular BMP modulators Tsg and BMPER to balance blood vessel formation
- Authors
- Heinke, J., Juschkat, M., Charlet, A., Mnich, L., Helbing, T., Bode, C., Patterson, C., and Moser, M.
- Source
- Full text @ J. Cell Sci.
|
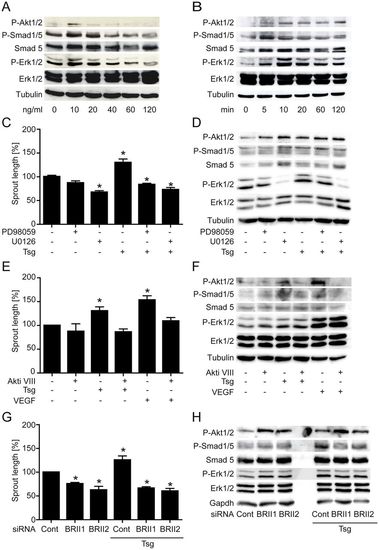
Recombinant Tsg protein induces concentration- and time-dependent intracellular signalling necessary for endothelial cell sprouting. (A,B) Dose and time dependence of intracellular signalling by Tsg in HUVECs. Western blot analyses were performed with the indicated antibodies. (C,E) Cumulative sprout length of HUVEC capillary-like structures from tube formation assays. (C) Tsg-stimulated sprouting was inhibited when Erk was blocked with signalling pathway inhibitors PD98059 (20μM) and U0126 (10μM) or (E) when Akt signalling was blocked with Akti VIII (2μM). Values are means ± s.e.m.; n = 3; *P<0.01 versus control. (D,F) Western blot analysis of Tsg-stimulated HUVECs with Erk inhibitors PD98059 (20μM) and U0126 (10μM) or Akt inhibitor Akti VIII (2μM) were performed with the indicated antibodies to determine the effects of inhibition of (D) Erk or (F) Akt phosphorylation on the other signalling pathways. (G,H) HUVECs were transfected with either of two BMPRII-specific siRNAs or scrambled siRNA control. (G) A tube formation assay was performed 48hours post-transfection and cumulative sprout length was measured. Values are means ± s.e.m.; n = 4; *P<0.01 versus siRNA control. (H) 48hours post-transfection cells were lysed and subjected to western blot analysis performed with the indicated antibodies. Representative western blots of one of three independent experiments are shown. |
|
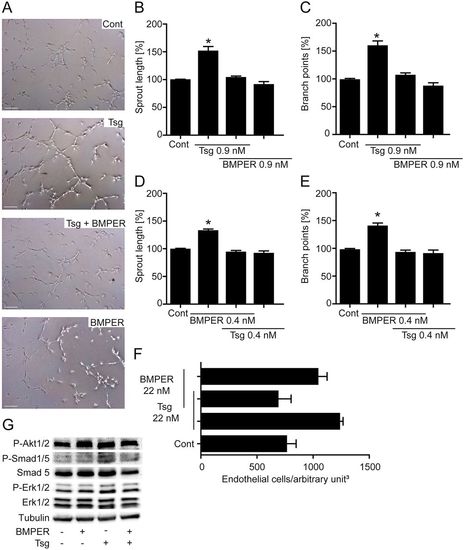
Simultaneous application of Tsg and BMPER in functional in vitro and in vivo angiogenesis assays abolishes induction of proangiogenic effects. (A–C) Tsg-stimulated HUVEC sprouting was inhibited when recombinant BMPER protein was added in equal amounts. (A) HUVECs were incubated with the indicated proteins and subjected to a tube formation assay. Representative micrographs of control, Tsg, BMPER and Tsg + BMPER simultaneous application are shown. Scale bars: 200µm. (B) Quantification of cumulative sprout length of capillary-like structures. (C) Number of branch points. (D,E) BMPER-stimulated HUVEC sprouting was inhibited when recombinant Tsg protein was added in equal amounts. (D) Quantification of cumulative sprout length of capillary-like structures. (E) Number of branch points. Values are means ± s.e.m.; n = 3; *P<0.001 versus control. (F) Quantification of mouse Matrigel plug assay with the indicated concentrations of recombinant Tsg and BMPER protein. Values are means ± s.e.m.; n = 3. (G) Western blot analysis of HUVECs stimulated with BMPER, Tsg and the combination of BMPER + Tsg (concentration = 0.9nM) performed with the indicated antibodies. Representative western blots of one of three independent experiments are shown. |
|
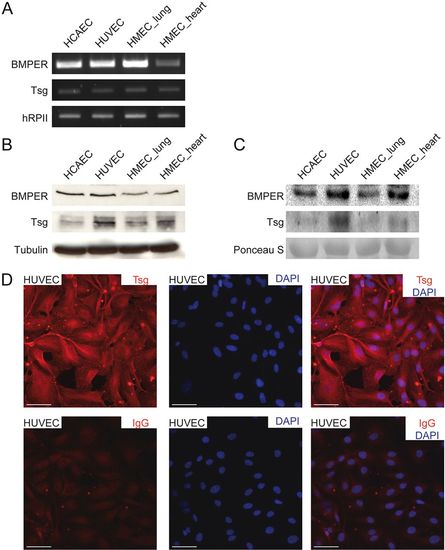
Tsg and BMPER expression in endothelial cells of different origin. (A–C) Expression of Tsg and BMPER in human coronary artery ECs (HCAECs), HUVECs and human microvascular ECs (HMECs) from lung and heart were analysed by (A) RT-PCR, (B) western blot analysis of cell lysates and (C) supernatants. (D) Localization of Tsg protein by immunocytochemistry in HUVECs (upper panel). Rat IgG2a was used as negative control (lower panel). Nuclei were stained with DAPI. Scale bars: 50 μm. |
|
Inhibition of Tsg in endothelial cells enhanced sprouting and signalling pathway activation. HUVECs were silenced for Tsg with either of two specific siRNAs or transfected with scrambled siRNA as control. (A,B) Six hours after siRNA transfection a collagen gel spheroid sprouting assay was performed. (A) Representative siRNA-transfected spheroids. Scale bars: 200μm. (B) Quantitative analysis of cumulative sprout length of spheroids. Values are means ± s.e.m.; n = 3; *P<0.001 versus siRNA control. (C) Migration was quantified 48hours post-transfection of HUVECs. Values are means ± s.e.m.; n = 3; *P<0.05 versus siRNA control. (D) Proliferation was determined by a BrdU assay. HUVECs were transfected in triplicate with the indicated siRNAs. 48hours post-transfection BrdU ELISA was performed. Values are means ± s.e.m.; n = 3; *P<0.001 versus siRNA control. (E) Mosaic spheroids were generated by mixing equal amounts of HUVECs transfected with either siRNA control and labelled with CMPTX (red) or siRNA targeted against Tsg (or control) and labelled with CFDA-SE (green). Confocal laser microscopy revealed equally mixed green and red sprouts under control conditions, whereas in partially Tsg-silenced spheroids, Tsg-silenced cells predominantly formed sprouts. Scale bars: 200μm. (F) Quantification of mosaic spheroids. (G) 48hours after siRNA transfection cells were lysed and subjected to western blot analysis, performed with the indicated antibodies. |
|
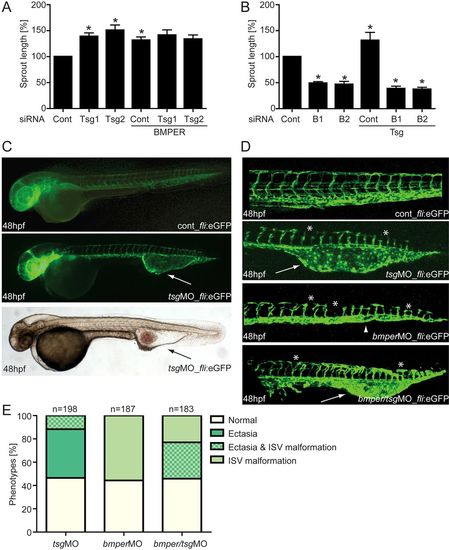
The BMP modulators BMPER and Tsg are necessary for proper angiogenesis in vitro and in zebrafish development. HUVECs were transfected with either Tsg- or BMPER-specific siRNAs, or with scrambled siRNA as a control. Thereafter, Tsg- or BMPER-silenced HUVECs were stimulated with BMPER and Tsg, respectively, and subjected to the tube formation assay. (A) Cumulative sprout length of Tsg-silenced HUVECs stimulated with BMPER and (B) cumulative sprout length of BMPER-silenced HUVECs stimulated with Tsg. Values are means ± s.e.m.; n = 3; *P<0.01 versus siRNA control. (C–E) Silencing of Tsg in zebrafish embryos causes malformations during blood vessel development. (C) Vasculature morphology of uninjected (top) and 0.125mM Tsg MO-injected (middle) tg(fli1:eGFP) fish embryos at 48hpf with formation of a large venous malformation (white arrows). (Bottom) Overall morphology of Tsg MO-injected zebrafish at 48hpf with blood accumulation in the ventral tail vein ectasia (black arrow). (D) Formation of the trunk vasculature in 48hpf tg(fli1:eGFP) fish embryos after injection of 0.125mM Tsg or 0.25mM BMPER MO or a combination of both. Tsg morphants displayed ventral tail vein ectasia (white arrows) and some disruption of ISVs and the DLAV (asterisks). In BMPER morphants disruption of ISVs and DLAV was more pronounced and in addition loss of the CVP (arrowhead) was observed. Double morphants displayed the malformations of both single Tsg/BMPER morphants. (E) Quantification of vascular defects in 48hpf tg(fli1:eGFP) Tsg and BMPER morphants. CVP, caudal vein plexus; DLAV, dorsal longitudinal anastomotic vessel; ISV, intersomitic vessels; hpf, hours post-fertilisation; MO, morpholino oligonucleotide. |