- Title
-
Knockdown of amyloid precursor protein in zebrafish causes defects in motor axon outgrowth
- Authors
- Song, P., and Pimplikar, S.W.
- Source
- Full text @ PLoS One
|
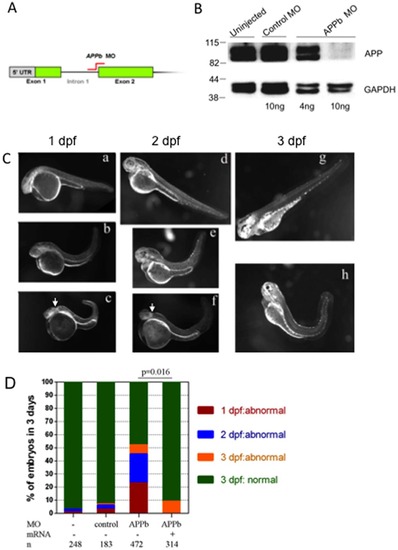
APPb is required for normal zebrafish embryonic development. (A) Schematic representation of APPb-MO blocking the mRNA splicing site between intron 1 and exon 2 (indicted in red) as used in this study. (B) Western blot analysis of APPb protein in zebrafish embryos at 2 dpf. The lower panel was probed with anti-GAPDH antibody. At 2 dpf, APPb protein migrated as a doublet at 98 KDa with stronger expressions in the un-injected group and control MO group. With the injection of 10 ng of APPb MO per embryo, APPb protein levels were not detected at 2 dpf. With the injection of 4 ng of APPb MO per embryo, weak expression of APPb protein migrating at 98 KDa was observed. (C) Morphological features of control embryos (a, d, g) and APPb morphant embryos (b, c, e, f, h). Lateral views (anterior to the left and dorsal at the top) of zebrafish embryos. The gross anatomical phenotype included a deformed body and a shortened and curved tail. In addition, defects in midbrain patterning were observed (arrows). 1 dpf: a, b, c; 2 dpf: d, e, f; 3 dpf: g, h. (D) APPb mRNA rescues the defective phenotype. There is little effect on normal embryonic development caused by the injection of control morpholino (APPb mis-match MO). Zebrafish embryos injected with 10 ng of APPb-MO expressed abnormal phenotypes at the 1 dpf (red), 2 dpf (blue) and 3 dpf (yellow) developmental stages. Embryos co-injected with 10 ng of APPb-MO and 350 pg APPb mRNA expressed normal phenotypes during embryogenesis. Statistical significance was measured comparing APPb-MO embryos and the co-injected embryos (p = 0.016, p<0.05, in 2-tailed paired t tests). PHENOTYPE:
|
|
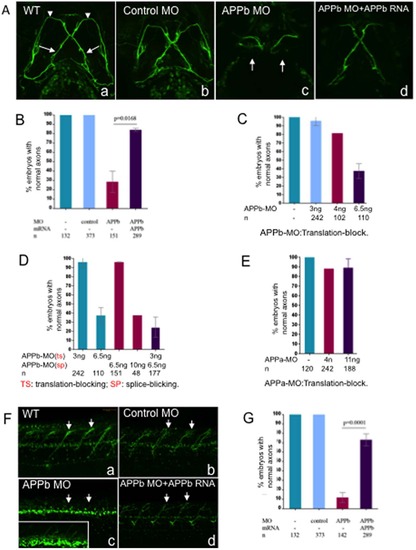
Embryos co-injected with APPb-MO and APPb mRNA rescued defective phenotypes of the Vp and VII and spinal cord nerve projections observed in APPb morphants. (A) Knockdown of APPb disrupts the projections of axons Vp and VII (3 dpf) when injected with APPb MO. Uninjected embryos and embryos injected with control MO did not show altered axonal outgrowth of neurons Vp and VII. Embryos injected with APPb-MO expressed axonal inhibition of axons Vp and VII (arrows). The defective phenotype was rescued by co-injection of APPb-MO and APPb mRNA. Ventral view; anterior, top. (B) Quantification of embryos expressing normal axonal outgrowth of neurons Vp and VII rescued through co-injection of APPb-MO (splice-block) and APPb mRNA. Uninjected embryos and embryos injected with control MO showed normal axonal growth of Vp and VII neurons. Embryos co-injected with 10 ng of APPb-MO and 350 pg APPb mRNA rescued the defected phenotype observed in the APPb morphant group. Statistical significance was established between APPb-MO embryos and co-injected embryos (p = 0.0168, p<0.05, in 2-tailed paired t test). (C) The translation-block MO of the APPb caused the identical defected phenotype on the axonal outgrowth of the Vp & VII neurons as the splice-block MO of the APPb; both were dose-dependent. (D) The morphants showed an identical defected phenotype on axonal outgrowth of Vp & VII neurons when co-injected with the translation-block MO (3 ng per embryo) of the APPb and the splice-block MO (6.5 ng per embryo) of the APPb at lower doses that did not produce a defective phenotype individually. (E) There was no defective phenotype of axonal outgrowth of Vp & VII neurons in the APPa morphants when injected with the translation-block MO against APPa. (F) APPb function is required for normal nerve outgrowth of the spinal cord. Lateral views of 3 dpf embryos (anterior is to the left, dorsal is at the top). Uninjected embryos and control embryos expressed normal motor nerve projections from the spinal cord to the myotomes (5–10 somites). Embryos injected with 10 ng of APPb-MO (splice-block) expressed severe motor neuron axon defects, including aberrant projections and decreased branching (arrows pointing at axon). Embryos co-injected with APPb-MO and APPb mRNA rescued the severe phenotype observed in the morphant group. The white rectangle in (c) is an amplification of the spinal cord neurons, which shows branching defects of the neurites in the APPb morphants. (G) Downregulation of APPb affects normal projection of motor nerves in the spinal cord. Compared with the control MO and uninjected groups, embryos injected with 10 ng APPb-MO (splice-block) showed significant axonal inhibition. Embryos co-injected with 10 ng of APPb-MO and 350 pg of APPb mRNA rescued the APPb morphant phenotype. Statistical significance was observed between morphant embryos and co-injected embryos (p = 0.0001, p<0.05 in 2-tailed paired t test). PHENOTYPE:
|
|
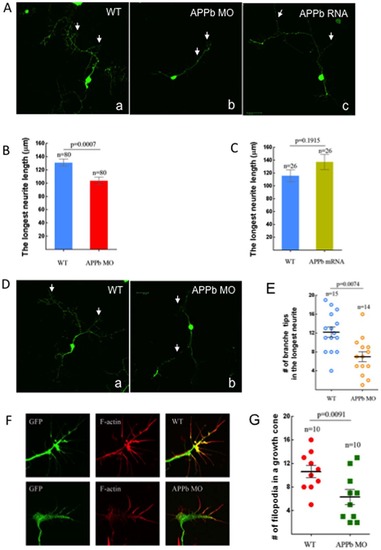
Neuronal cell cultures derived from embryos injected with APPb-MO had decreased neurite length. (A) Neuronal culture from control embryos and APPb mRNA (350 pg) over-expression embryos exhibited on average 3 primary branches with several secondary branches. Neuronal cultures from embryos injected with 8 ng of APPb-MO exhibited on average 1 primary branch with many secondary branches. 8 ng of APPb-MO is sufficient to inhibit neruite growth, so embryos were injected with 8 ng of the APPb MO instead of the 10 ng to limit the potential for toxicity. The neurons were cultured for 2 days before fixing. (B) Down-regulation of APPb in cultured neurons affected normal neurite growth. The neurons from the embryos injected with 8 ng of APPb-MO showed a decrease in neurite length compared to neurons from the control embryos (un-injected). The average neurite length of a control neuron was about 130 µm, compared to only about 100 µm in the morphant embryos. Statistical significance was observed between control neurons and APPb knockdown neurons (8 ng APPb MO) (p = 0.0007, p<0.05 in two-tailed paired t-test). (C) Quantifying the effects of APPb mRNA in cultured neurons. No significant difference was observed in neurite length between control and APPb mRNA over-expression cultured neurons. Control cultured neurons expressed a shorter neurite length compared to APPb mRNA over-expression neurons. No statistical significance was observed (p = 0.1915, p>0.05 in two tailed paired t-test). (D) There were fewer branches of neurites in the APPb knockdown neurons (8 ng of APPb MO) than in control neurons (WT); only branches with more than 5 µm were counted. The neurons were cultured for 2 days. (E) Down-regulation of APPb in cultured neurons affected the number of branch tips. Compared to control neurons, APPb-MO cultured neurons showed a decreased number of branch tips of the longest neurite. Statistical significance was observed between control and APPb-MO cultured neurons (p = 0.0074, p<0.05 in two tailed paired t-test). (F) Down-regulation of APPb in cultured neurons affected the morphology and projection of growth cones and filopodia. APPb knockdown neurons expressed abnormal growth cone morphology and a decreased number of filopodia (20 hour neuron cultures). (G) Reduced number of filopodia in APPb knockdown cultured neurons. Compared to control cultured neurons, APPb-MO (8 ng per embryo) cultured neurons expressed a reduced number of filopodia. Statistical significance was observed between control and APPb cultured neurons (p = 0.0091, p<0.05 in two tailed paired t-test). |
|
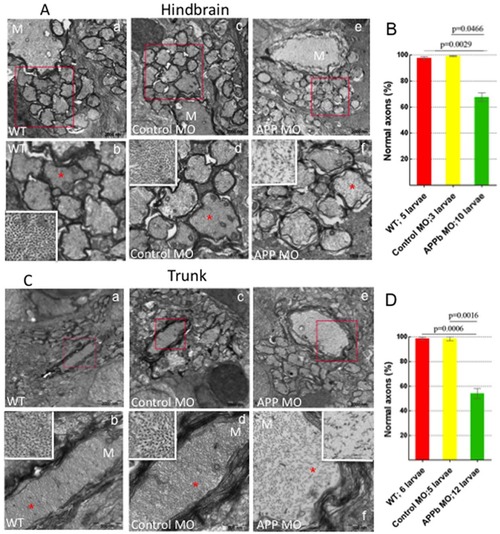
Analysis of Transmission Electron Microscopy images of axons in zebrafish hindbrain and trunk regions (5 dpf). (A) Transmission Electron Microscopy (TEM) images of axons in zebrafish hindbrain. Compared to uninjected and control MO-injected embryos, zebrafish embryos injected with APPb-MO (8 ng) expressed a decreased density and disorganization of the cytoskeleton in both the Mauthner (M) axons and the axons around the M axon. Panels b, d, and f are amplifications of the boxed areas in panels a, c and e, respectively. The white rectangles in panels b, d, and f are amplifications of the areas marked with red 5-point stars. (B) Quantifying the defects of axonal cytoskeletal morphology in the hindbrain of APPb morphant embryos. At 5 dpf, zebrafish embryos injected with 8 ng of APPb-MO showed a disruption in axon cytoskeletal dynamics in the hindbrain region. For the TEM experiment, the embryos were injected with 8 ng of the APPb MO instead of 10 ng. The embryos that were injected with 8 ng of the APPb MO experienced disorganization of the axonal cytoskeleton; the cytoskeleton of the embryos that were injected with 10 ng of APPb MO experienced severely defects in or loss of axons. Statistical significance was observed comparing uninjected (263 axons) and morphant (662 axons) larvae (p = 0.0029, p<0,05 in two-tailed paired t-test). Statistical significance was also observed between control MO (217 axons) and morphant larvae (p = 0.0466, p<0.05 in two tailed paired t-test). (C) TEM images of axons at in the zebrafish trunk section. Compared to uninjected and control MO-injected larvae, zebrafish larvae injected with 8 ng of APPb-MO expressed a decrease in axonal density and had defects in cytoskeletal organization of axons, including the M axon. Panels b, d, and f are amplifications of the boxed areas in panels a, c and e, respectively. The white rectangles in panels b, d and f are amplifications of the areas marked with red 5-point stars. (D) Quantifying defects of axonal cytoskeletal morphology in the trunk region of APPb morphant embryos. At 5 dpf, zebrafish embryos injected with 8 ng of APPb MO expressed an abnormal phenotype in axonal cytoskeletal organization in the trunk region. Statistical significance was observed comparing uninjected (217 axons) and morphant (562 axons) larvae (p = 0.0006, p<0.05 in two tailed paired t-test). Statistical significance was also observed between control MO (212 axons) and morphant larvae (p = 0.0016, p<0.05 in two tailed paired t-test). PHENOTYPE:
|
|
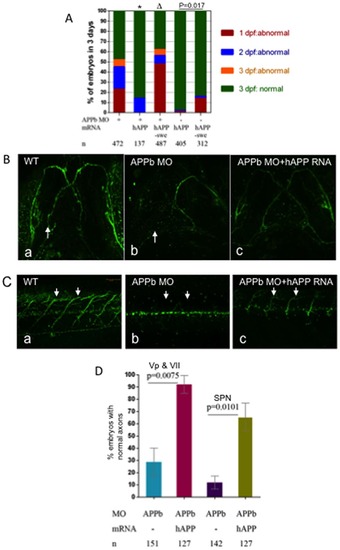
hAPP695 (hAPP), but not hAPP-swe, effectively rescued defects in axonal outgrowth in APPb-MO morphants. (A) Co-injection of hAPP695 mRNA rescued APPb morphant embryonic morphology. As depicted, embryos were inspected over a period of 3 days. Co-injection of full length human APP695 mRNA rescued the defective phenotype observed in the APPb morphant embryos (*, p = 0.026, p<0.05 in two tailed paired t-test). In contrast, human APP-Swedish mutation (Δ, p = 0.5241, p>0.05) and AICD mRNA failed to rescue the APPb morphant phenotype. Combined injections (10 ng of APPb-MO and 350 pg of AICD mRNA) caused more severe deficits compared to injecting APPb-MO alone. (B) Co-injection of hAPP695 mRNA rescued axonal outgrowth of motor neurons Vp and VII of the APPb morphant. Zebrafish embryos co-injected with full length human APP695 rescued the APPb morphant axonal outgrowth phenotype of motor neurons Vp and VII (arrow). (C) Embryos co-injected with hAPP695 rescued the defective phenotype of motor axons in the spinal cord in APPb morphant embryos. Uninjected embryos expressed normal motor neuron projections from the spinal cord to the myotomes (5–10 somites). Embryos injected with (10 ng) APPb-MO expressed severe motor neuron axonal defects including aberrant projections and decreased branching. Embryos co-injected with APPb-MO and hAPP695 mRNA had a rescued phenotype compared to the morphant group. Lateral views of 3 dpf embryos (anterior is to the left, dorsal is at the top). (D) Quantification of embryos expressing normal axonal outgrowth of neurons Vp and VII and spinal cord rescued through co-injection of hAPP695 mRNA. Injection of 10 ng of APPb-MO caused abnormal axonal outgrowth of motor neurons Vp and VII in 75% of embryos. Co-injection with mRNA encoding full-length human APP695 overwhelmingly rescued the APPb morphant phenotype (p = 0.0075, p<0.05 in two tailed paired t-test) (3 dpf). Embryos injected with 10 ng APPb-MO showed significant inhibition of spinal cord axon outgrowth. Embryos co-injected with 10 ng of APPb-MO and 350 pg of hAPP695 mRNA rescued the APPb morphant phenotype (p = 0.0101, p<0.05 in two tailed paired t-test). |
|
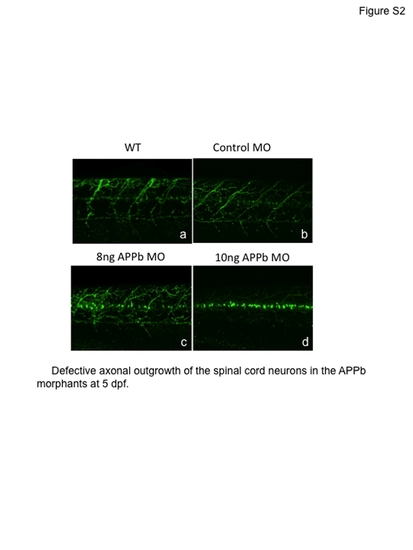
Embryos injected with APPb-MO (splice-block) still expressed motor neuron axon defects at 5 dpf. PHENOTYPE:
|
|
Morphological features of normal (a, c, e) and abnormal (b, d, f) embryos injected with APP mRNA and its truncated mRNA. |