- Title
-
Epidermal growth factor differentially regulates activin subunits in the zebrafish ovarian follicle cells via diverse signaling pathways
- Authors
- Chung, C.K., and Ge, W.
- Source
- Full text @ Mol. Cell. Endocrinol.
|
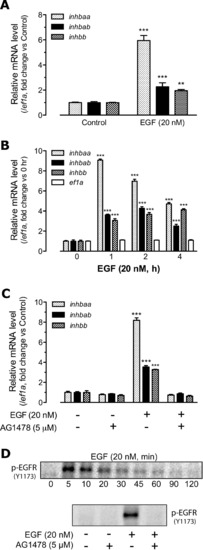
Effects of recombinant human EGF (20 nM) on the expression of activin subunits inhbaa, inhbab and inhbb in the zebrafish ovary. (A) Effect of EGF on activin subunit expression in intact follicles. Follicles were treated with 20 nM EGF for 2 h before sampling for RNA extraction. (B) Time course of EGF effects on activin subunit expression in cultured follicle cells. The total treatment time was 4 h and EGF was added at different times towards the end of the treatment. The expression levels of activin subunits were normalized to the housekeeping gene ef1a. (C) Blockade of EGF effects by EGFR inhibitor AG1478. Zebrafish follicle cells were pre-treated with 5 μM AG1478 for 30 min prior to treatment with 20 nM EGF for 2 h. (D) EGF-stimulated phosphorylation of EGFR. The cells were sampled 5 min after EGF treatment at 20 nM. The relative mRNA levels were determined by real-time qPCR and normalized to the housekeeping gene ef1a. The values are the mean ± SEM (n = 3–4). **P < 0.01; ***P < 0.001 vs. respective control of each gene. Each graph is representative of three independent experiments. |
|
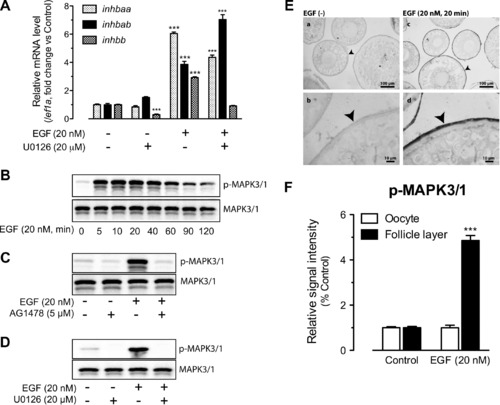
Involvement of MAPK3/1 in EGFR signaling and EGF-stimulated activin subunit expression. (A) Effects of U0126 (MEK1/2 inhibitor) on EGF-stimulated expression of inhbaa, inhbab and inhbb in cultured follicle cells. The cells were treated with EGF at 20 nM for 2 h. The expression values are the mean ± SEM (n = 3–4). ***P < 0.001 vs. respective control of each gene. The graph is representative of three independent experiments. (B) Time course of EGF-induced MAPK3/1 phosphorylation (p-MAPK3/1) in the follicle cells. (C) Blockade of EGF-induced MAPK3/1 phosphorylation by EGFR inhibitor AG1478. (D) Blockade of basal and EGF-induced MAPK3/1 phosphorylation by MEK1/2 inhibitor U0126. The follicle cells were pre-treated with the inhibitors for 30 min before treatment with EGF for 5 min. (E) Immunohistochemical staining for EGF-induced MAPK3/1 phosphorylation in intact follicles. The follicles were treated with (20 nM) or without EGF for 20 min before sampling for fixation, sectioning and IHC staining. No signal was detected in the control follicles without EGF treatment (a and b), whereas strong signal of MAPK3/1 phosphorylation was detected in the somatic follicle layer (arrow head) but not the oocyte (c and d). (F) Semi-quantitative measurement of immunohistological staining for p-MAPK3/1 in the follicle layer and oocyte compartments of intact follicles. MAPK3/1 phosphorylation level increased in follicle layer but not the oocyte in response to EGF treatment. The values are the mean ± SEM (n = 4). ***P < 0.001 vs. control. |
|
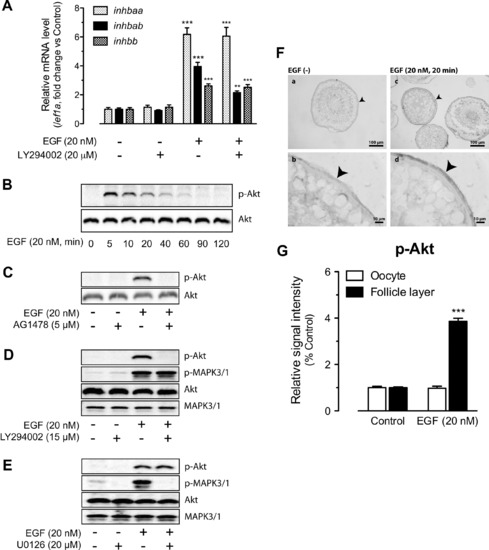
Involvement of PI3K/Akt in EGFR signaling and EGF-stimulated activin subunit expression. (A) Effects of LY294002 (PI3K inhibitor) on EGF-stimulated expression of inhbaa, inhbab and inhbb in cultured follicle cells. The cells were treated with EGF at 20 nM for 2 h. The expression values are the mean ± SEM (n = 3–4). **P < 0.01; ***P < 0.001 vs. respective control of each gene. The graph is representative of four independent experiments. (B) Time course of EGF-induced Akt phosphorylation (p-Akt) in the follicle cells. (C) Effects of EGFR inhibitor AG1478 on basal and EGF-induced Akt phosphorylation. (D) Effects of PI3K inhibitor LY294002 on Akt and MAPK3/1 phosphorylation. (E) Effects of MEK1/2 inhibitor U0126 on Akt and MAPK3/1 phosphorylation. The follicle cells were pre-treated with the inhibitors for 30 min before treatment with EGF for 5 min. (F) Immunohistochemical staining for EGF-induced Akt phosphorylation in intact follicles. The follicles were treated with (20 nM) or without EGF for 20 min before sampling for fixation, sectioning and IHC staining. No signal was detected in the control follicles without EGF treatment (a and b), whereas signal of Akt phosphorylation was detected in the somatic follicle layer (arrow head) but not the oocyte (c and d). (G) Semi-quantitative measurement of immunohistological staining for p-Akt in the follicle layer and oocyte compartments of intact follicles. p-Akt level increased in the follicle layer after EGF treatment. The values are the mean ± SEM (n = 4). ***P < 0.001 vs. control. |
|
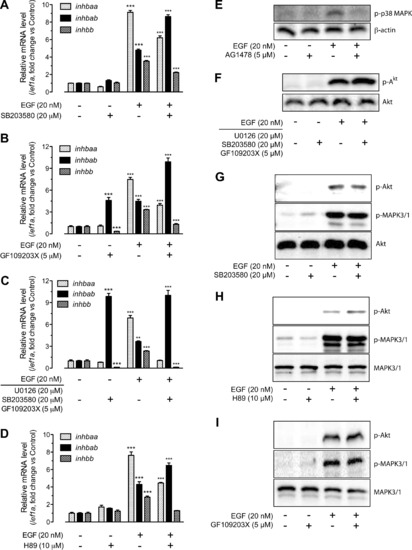
Involvement of p38 MAPK, PKC and PKA in EGFR signaling and EGF-stimulated activin subunit expression. (A) Effects of SB203580 (p38 MAPK inhibitor) on EGF-stimulated expression of inhbaa, inhbab and inhbb in cultured follicle cells. The cells were treated with EGF at 20 nM for 2 h and the inhibitor was added 30 min earlier. (B) Effects of GF109203X (PKC inhibitor) on EGF-stimulated expression of inhbaa, inhbab and inhbb in cultured follicle cells. (C) Combined effects of U0126, SB203580 and GF109203X on the expression of activin subunits. The cells were pre-treated with the three inhibitors for 30 min followed by treatment with EGF at 20 nM for 2 h. (D) Involvement of PKA in basal and EGF-stimulated expression of activin subunits. The follicle cells were treated with EGF at 20 nM for 2 h in the presence or absence of H89 (PKA inhibitor), which was added 30 min earlier. The expression values are the mean ± SEM (n = 3–4). **P < 0.01; ***P < 0.001 vs. respective control of each gene. Each graph is representative of four independent experiments. (E) EGF-induced p38 MAPK phosphorylation (p-p38 MAPK) in the follicle cells. β-actin was used as the loading control. The phosphorylation could be abolished by EGFR inhibitor AG1478. (F) Effects of U0126, SB203580 and GF109203X on basal and EGF-induced Akt phosphorylation in cultured follicle cells. (G–I) Effects of SB203580, H89 and GF109203X on basal and EGF-stimulated Akt and MAPK3/1 phosphorylation. The zebrafish follicle cells were pre-treated with each inhibitor for 30 min before treatment with EGF for 5 min. |
Reprinted from Molecular and Cellular Endocrinology, 361(1-2), Chung, C.K., and Ge, W., Epidermal growth factor differentially regulates activin subunits in the zebrafish ovarian follicle cells via diverse signaling pathways, 133-142, Copyright (2012) with permission from Elsevier. Full text @ Mol. Cell. Endocrinol.