- Title
-
Zebrafish cadherin-11 participates in retinal differentiation and retinotectal axon projection during visual system development
- Authors
- Clendenon, S.G., Sarmah, S., Shah, B., Liu, Q., and Marrs, J.A.
- Source
- Full text @ Dev. Dyn.
|
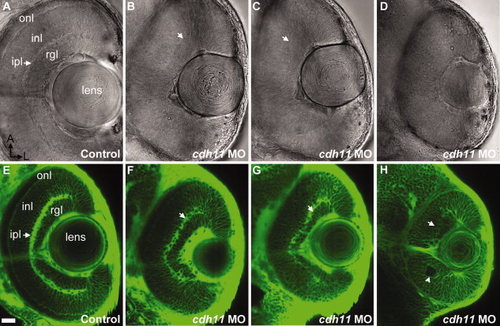
Eyes of cdh11 morphants are small and lamina organization is disrupted at 2 days postfertilization (dpf). A–H: Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO; control, A and E) or cdh11 MO (B–D,F–H). A–D: Ventral views eyes of living embryos were imaged using differential interference contrast microscopy (A–D). E–H: BODIPY-ceramide labeled living 2 dpf embryos were imaged using confocal microscopy. Anterior is up and lateral is right. All the major layers characteristic of the adult retina are seen in control embryos, including the retinal ganglion cell layer (rgl), inner plexiform layer (ipl; arrow), the inner nuclear layer (inl), and the outer nuclear layer (onl). B–D,F–H: Increasing severity of Cdh11 loss-of-function phenotype were observed: B and F, in slightly affected embryos, eyes were similar sizes but retinal lamina were reduced; C and G, in moderately affected embryos, the eyes were smaller in size, the inner plexiform layer was sparse, the distance between the inner plexiform layer and the lens was not uniform, and the outer nuclear layer was sparse or absent; D and H, eyes and lenses of severely affected embryos were small, rosettes were evident (arrowhead) and laminae nearly absent. Scale bar = 20 μm. PHENOTYPE:
|
|
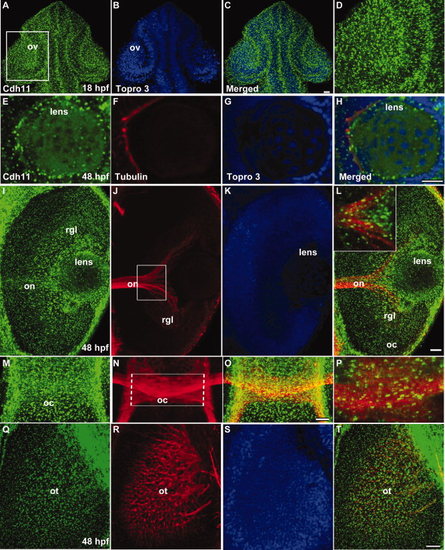
Distribution of Cdh11 in the developing lens, retina, and retinotectal projections. Laser scanning confocal microscope images are shown. Panels A–H, L (inset), and P are single optical sections, and other images are projection images of several optical sections to show neural projections through the tissues. Embryos at 18 hours postfertilization (hpf) were double labeled using antibodies that specifically recognize Cdh11 (green, panels A, C, and D) and TO-PRO-3 (nuclear stain, blue, panels B and C). Cdh11 and nuclear label images were merged (panel C). The boxed region in panel A contains the optic vesicle (ov), showing details of the retina and lens, are shown in panel D. A–D: Dorsal views are shown, and anterior is up. Embryos at 48 hours postfertilization (hpf) were triple labeled using antibodies that specifically recognize Cdh11 (green, panels E, I, M, and Q); antibodies that specifically recognize acetylated tubulin (red, panels F, J, N, and R); and TO-PRO-3 that labels nuclei (blue, panels G, K and S). Merged images (panels H, L, O, P, and T) are shown for comparison of expression patterns. Cdh11 was detected in the retina and lens at 18 and 48 hpf. E–H,M–T: Ventral views are shown, and anterior is up. I–L: Anterior is left, and lateral is up. Acetylated tubulin labels neuronal processes, including the optic nerve (on, panels J and L); the optic chiasm (oc, panels N, O and P); and the retinotectal projections arriving in the optic tectum (ot, panels R and T). Views of the eye (I and L) and brain (M, O, P, Q, and T) illustrate Cdh11 staining is associated with the optic nerve and optic chiasm. Scale bars = 20 μm. EXPRESSION / LABELING:
|
|
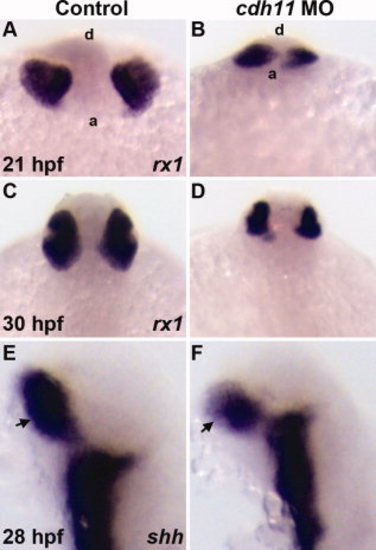
Expression of early retinal specification markers in cdh11 morphant embryos. Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO, Control) or cdh11 MO. Whole-mount in situ hybridization labeling of embryos at different stages was performed using probes for markers retinal specification. Probes are shown in the bottom right corner of each panel. A,B:rx1 labeling of 21 hours postfertilization (hpf) control and cdh11 morphant embryos. Anterior views from a ventral perspective; a, anterior embryo border; d, dorsal surface. rx1 labeling was detected in control (A) and cdh11 morphant embryos (B). C,D:rx1 labeling was strongly detected in 30 hpf control and cdh11 morphant embryos. Dorsal view of 30 hpf embryos: anterior is top. E,F: Expression of shh, a marker of retinal stalk (arrows) differentiation, was strongly detected in 28 hpf control and cdh11 morphant embryos; lateral views, anterior is up. PHENOTYPE:
|
|
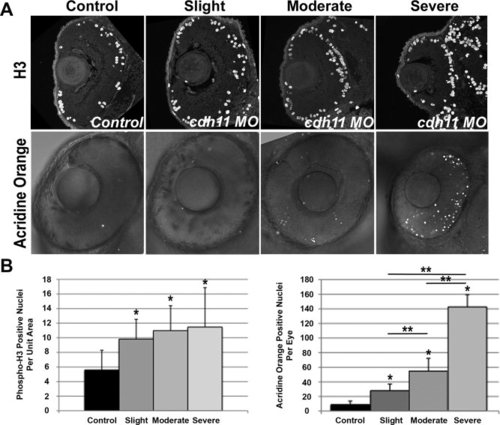
Microphthalmia is due to increased cell death in cdh11 knockdown embryos. Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO, Control) or cdh11 MO (Slight, Moderate, Severe). A: Mitotic cells were visualized in eyes of 48 hours postfertilization (hpf) embryos using histone-H3 immunostaining. Most dividing cells were found in peripheral lamina of the retina, in both control and knockdown embryos. Apoptosis was detected using acridine orange staining in eyes of 48 hpf embryos. Fluorescent nuclei were detected using confocal microscopy, and fluorescent images were overlayed on differential interference contrast transmitted light images. Increasing numbers of fluorescent nuclei were detected in embryos with increasing severity of cdh11 knockdown phenotype. Anterior is up, and lateral is left. B: Quantitative analysis of H3-positive nuclei per unit area (arbitrary units) showed a significant increased mitosis rate in cdh11 knockdown eyes. Graph shows data separated into slightly, moderately and severely affected categories using criteria outlined in the text. Error bars represent standard deviation of the mean. Control, n = 10; slight, n = 9; moderate, n = 10; severe, n = 9. Analysis of variance (ANOVA) comparisons of control vs. pooled data from all groups and control vs. slight, moderate or severe groups were significant (*P < 0.05), but the mitosis rate differences between slight, moderate or severe groups were not statistically significant. Quantitative analysis of acridine orange stained nuclei showed a significant increased apoptosis rate in cdh11 knockdown eyes. Graph shows data separated into slightly, moderately and severely affected categories using criteria outlined in the text. Error bars represent standard deviation of the mean. Control, n = 10; slight, n = 12; moderate, n = 8; severe, n = 5. ANOVA comparisons of control vs. pool data from all groups and control vs. slight, moderate or severe groups were significant (*P < 0.001), and the apoptosis rate differences between slight, moderate or severe groups were statistically significant (**P < 0.001). PHENOTYPE:
|
|
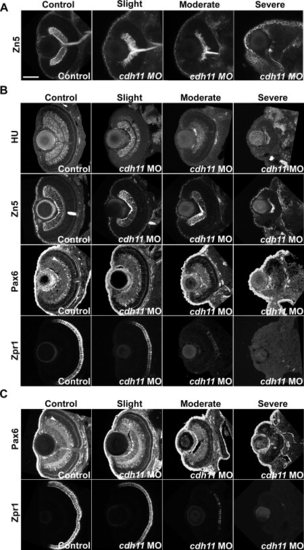
cdh11 knockdown inhibits retinal differentiation. A–C: Zebrafish embryos were injected with standard control morpholino oligonucleotide (MO, Control columns) or cdh11 MO (Slight, Moderate, and Severe columns). A: In 2 day postfertilization (dpf) embryos, retinal ganglion cells were visualized using whole-mount zn-5 monoclonal antibody staining and confocal imaging. Ventral view projections of sections through the optic nerve show retinal ganglion cells and their axon processes. In cdh11 morphant embryos, the retinal ganglion cell layer was progressively reduced in thickness and numbers of ganglion cells detected. B: In 3 dpf embryos, cryostat sections were immunolabeled using antibodies that detect retinal layers: HuC/D (HU), which detects retinal ganglion cells and inner nuclear layer neurons; zn-5 (Zn5), which detects retinal ganglion cells and their axons; Pax6 (Pax6), which detects cells in the ganglion cell layer and brightly labeled amacrine cells within the inner nuclear layer (arrowhead in Control image); and zpr-1 (Zpr1), which detects double cone photoreceptors. Cdh11 loss-of-function results in a progressive reduction in immunostaining markers for differentiated cell types in the retina. C: In 4 dpf embryos, cryostat sections were immunolabeled using antibodies that detect retinal layers: Pax6 (Pax6), which detects a variety of cells including brightly labeled amacrine cells within the inner nuclear layer (arrowhead in Control image); and zpr-1 (Zpr1), which detects double cone photoreceptors. The older embryos maintain the Cdh11 loss-of-function phenotype, showing reduced labeling of differentiated cell types. Scale bar = 100 μp> |
|
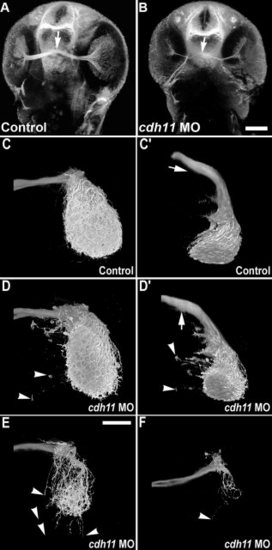
Retinotectal projections are reduced in cdh11 knockdown embryos. In 2 days postfertilization embryos, acetylated tubulin labeling detects retinal ganglion cell processes (optic nerve) and the optic chiasm (arrow). Image volumes were collected using 2-photon microscopy. A: Control embryo. B:cdh11 morphant embryo. In cdh11 knockdown embryos, retinal ganglion cell processes were present, but the optic nerve was thinner. C–F: Anterograde labeling of retinotectal projections are reduced in cdh11 knockdown embryos. Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO, C and C2) or cdh11 MO (D,D2,E,F). Embryos were fixed at 3 days postfertilization (dpf), and retinotectal projections were traced by injecting eyes with the lipophilic dye DiI (1,1-dioctadecyl-3,3,3,3-tetramethylindocarbo- cyanine perchlorate), and image volumes of the retinotectal projections were collected using 2-photon microscopy. In both controls and cdh11 knockdowns, retinal ganglion cell axons project toward the contralateral optic tectum. In control embryos (C,C2, where C2 is a rotation of the volume shown in C), upon reaching the optic tectum, retinal ganglion cells projections arborized extensively within the tectal neuropil. In slightly affected cdh11 knockdowns (D,D2), the optic nerve arborized extensively within the tectal neuropil, but there were projections that extended beyond the tectal neuropil (arrowheads). Also, there ectopic projections were detected at the optic chiasm (arrows in D and D2, where D2 is a rotation of the volume shown in D). In moderately affected embryos (E) and severely affected embryos (F), retinotectal projections were reduced, but those that reached the tectum arborized within the neuropil. In these embryos, there were also frequently projections that extended beyond the neuropil (arrowheads). Scale bars = 100 μm in A,B, 50 μm in C–F. PHENOTYPE:
|