- Title
-
Role of zebrafish lbx2 in embryonic lateral line development
- Authors
- Chen, X., Lou, Q., He, J., and Yin, Z.
- Source
- Full text @ PLoS One
|
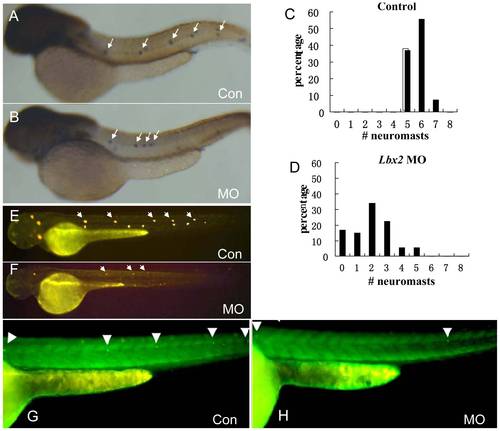
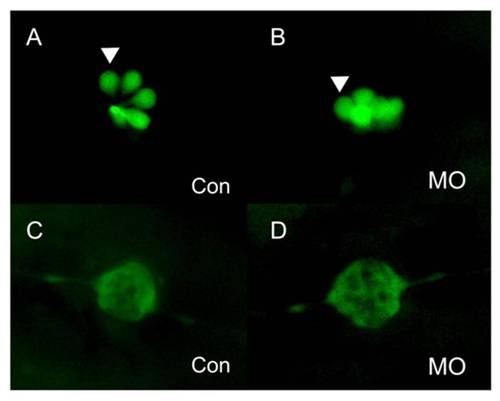
PLL cells in the newly deposited neuromasts of lbx2 morphants appear normal. (A–B) Hair cells of PLL neuromasts labeled with GFP in the SqET4 transgenic zebrafish line. The pattern and numbers of PLL hair cells in newly deposited neuromasts was similar in control embryos (A) and lbx2 morphants (B) at 48 hpf. (C, D) Fluorescence images of SqET10 embryos indicating that the supporting cells and lateral line nerve in newly deposited neuromasts of embryos injected with control MO (C) or lbx2 MO (D) are similar at 48 hpf. The white arrowhead indicates HCs in deposited neuromasts. |
|
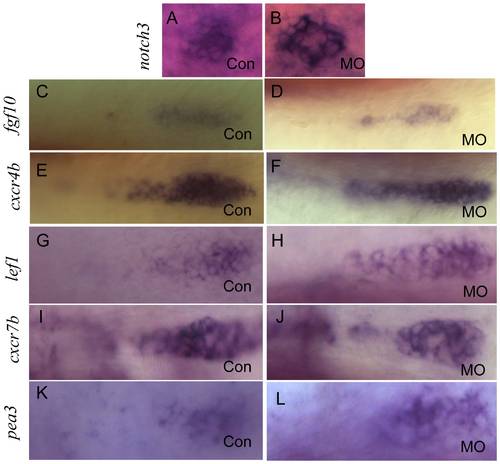
Integration of the migrating PLL primordia is not affected in lbx2 morphants. (A, B) Whole mount in-situ hybridization for notch3 indicating that compared to control embryos (A), the PLL hair cells of deposited neuromasts are not perturbed in lbx2 morphants (B). (C, D) Compared control embryos (C), no difference in fgf10 expression was evident in the protoneuromast rosettes of newly deposited neuromasts in lbx2 morphants (D). (E, F) Compared to control embryos (E), there was no difference in cxcr4b expression in the protoneuromast rosettes of newly deposited neuromasts in lbx2 morphants (F). (G, H) Compared to control embryos (G), there were no appreciable difference in lef1 expression in the migrating PLL primordia in lbx2 morphants (H). (I, J) Compared to control embryos (I), there were no appreciable difference in cxcr7b expression in the migrating PLL primordia in lbx2 morphants (J). (K, L) Compared to control embryos (K), there were no appreciable difference in cxcr7b expression in the migrating PLL primordia in lbx2 morphants (L). Embryos used in the assay were at 48 hpf stage. |
|
Disassociation of the PLL primodium and PLL nerve in lbx2 morphants. (A, B) Labeling of the PLL nerve using an anti-acetylated α-tubulin antibody, indicating that the PLL nerve grew correctly in both control embryos (A) and lbx2 morphants (B). (C, D) Immunohistochemical detection of the PLL nerve using an anti-acetylated alpha tubulin antibody to label PLL neuromasts and in situ hybridization using a cldnb antisense probe to label the primordia, revealing disassociation of the primodium and nerve in lbx2 morphants (D), but not in control embryos (C). White arrows indicate the PLL nerve, black arrows show the deposited neuromasts and the black asterisk indicates the stalled primordium. Embryos used in the assay were at 48 hpf stage. |
|
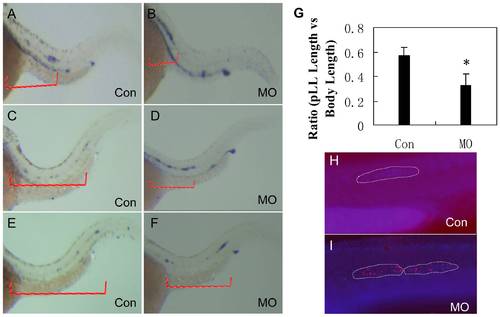
Migration of the PLL primordium is altered in lbx2 morphants. Migrating PLL atoh1a-expressing hair cells in control embryos (A, C, E) and lbx2 morphants (B, D, F) at 36 hpf (A, B), 40 hpf (C, D) and 48 hpf (E, F). (G) Analysis of the distance of PLL primordium migration in 48 hpf lbx2 morphants and controls. (H, I) TUNEL assay coupled with DAPI staining, indicating elevated cell death in the slowly migrating PLL primordium and deposited neuromasts of lbx2 morphants (I) compared with a similar region of the migrating PLL primordium in control embryos (H) at 36 hpf. Violet dots mark the migrating PLL primordium labeled with DAPI, stippled white lines indicate the zone of the PLL primordium and red dots indicate TUNEL positive cells. |
|
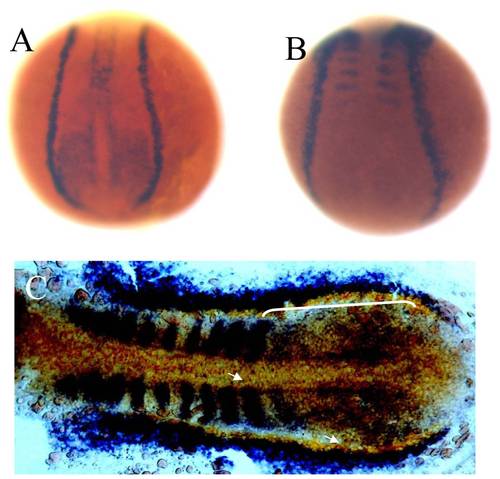
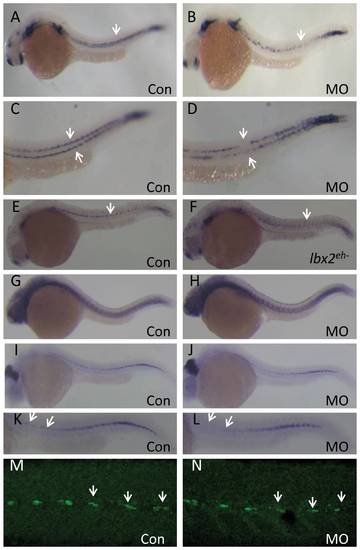
Overlapping expression domains of zebrafish lbx2 and sdf1a during early development. (A, B) Expression patterns of lbx2 (A) and sdf1a (B) at the tail bud stage (dorsal toward the top view). (C) lbx2 (blue) and sdf1a (red) have an overlapping expression zone in the posterior lateral mesoderm and adaxial cells at the 10-somite stage (dorsal view). White bracket and arrows in C mark the overlapping zone of gene expression. |
|
Abrogation of zebrafish lbx2 results in a defective sdf1a expression pattern in the horizontal myoseptum. (A, B) Compared with control embryos (A), a weak and discontinuous sdf1a expression pattern is observed in the horizontal myoseptum of lbx2 morphants at 24 hpf (B; lateral view). (C, D) Magnified views of the expression patterns shown in A (C) and B (D). (E, F) Compared with gfp mRNA-injected control embryos (E), injection of lbx2eh- mRNA resulted in a defective sdf1a expression at 24 hpf (F). (G, H) Similar expression pattern of tenascin C, a marker of the horizontal myoseptum, in control embryos (G) and lbx2 morphants (H) at 30 hpf. (I, J) Analysis of eng2a positive muscle pioneer cells in control embryos (I) and lbx2 morphants (J) at 30 hpf. (K, L) Magnified views of the expression patterns shown in I (K) and J (L), showing decreased numbers of eng2a positive muscle pioneer cells in lbx2 morphants (L) compared to control embryos (K). (M, N) Compared with control embryos (M), the numbers of 4D9 positive muscle pioneer cells was slightly reduced in lbx2 morphants (N) at 30 hpf. White arrows indicate the hybridization or immunostaining signals. All images are lateral views. |
|
Efficiency of the lbx2 morpholino. (A) A test lbx2-EGFP construct was created containing 60 bp of the 5′ UTR and the first 66 amino acid coding sequence of lbx2 cDNA fused to the N-terminus of EGFP, driven by the CMV promoter. The sequence of lbx2 MO is complementary to the 1–24 bp region of zebrafish lbx2 cDNA. (B) Live embryos at the 50% epiboly stage. Embryos co-injected with 25 ng lbx2-EGFP DNA and 5 ng control MO expressed green fluorescent fusion protein (left), which was inhibited by co-injection of 2 ng lbx2 MO (right). (C) Translation of lbx2-EGFP in live embryos was inhibited by co-injection of lbx2 MOs. (D) The lbx2 protein level in lbx2-MO-injected embryos was drastically lower than control MO-injected embryos at 30 hpf (E). Absence of MyoD expression in the pectoral fin bud of lbx2 morphants at 48 hpf, which could be rescued by co-injection of lbx2 mRNA. Arrowhead indicates MyoD expression in the pectoral fin bud area. (F–I) Injection of lbx2eh- mRNA dramatically inhibited MyoD expression in pectoral fin muscle precursors at 30 hpf (G) and 36 hpf (I), compared to gfp mRNA-injected control embryos (F, H). |
|
PLL cells in the newly deposited neuromasts of lbx2 morphants appear normal. (A–B) Hair cells of PLL neuromasts labeled with GFP in the SqET4 transgenic zebrafish line. The pattern and numbers of PLL hair cells in newly deposited neuromasts was similar in control embryos (A) and lbx2 morphants (B) at 48 hpf. (C, D) Fluorescence images of SqET10 embryos indicating that the supporting cells and lateral line nerve in newly deposited neuromasts of embryos injected with control MO (C) or lbx2 MO (D) are similar at 48 hpf. The white arrowhead indicates HCs in deposited neuromasts. |