- Title
-
Phosphorylation of the Zebrafish M6Ab at Serine 263 Contributes to Filopodium Formation in PC12 Cells and Neurite Outgrowth in Zebrafish Embryos
- Authors
- Huang, K.Y., Chen, G.D., Cheng, C.H., Liao, K.Y., Hung, C.C., Chang, G.D., Hwang, P.P., Lin, S.Y., Tsai, M.C., Khoo, K.H., Lee, M.T., and Huang, C.J.
- Source
- Full text @ PLoS One
|
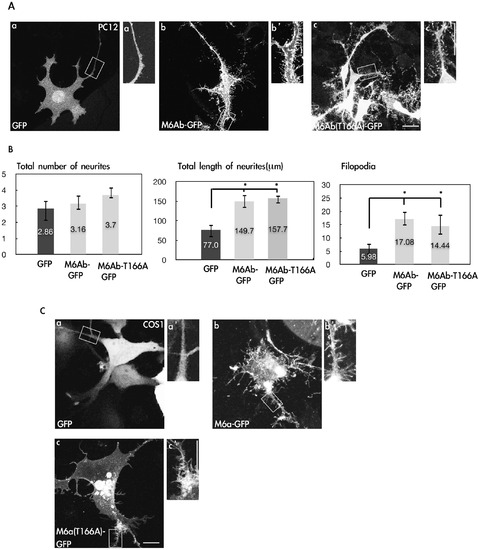
Overexpression of zM6Ab can induce neurite outgrowth and filopodia in PC12 and COS-1 cells. (A) PC12 cells were transfected with the control pcDNA3-GFP or pcDNA3-M6Ab-GFP or pcDNA3-M6Ab-T166A-GFP plasmids. Twenty-four hours after transfection, cells were treated with nerve growth factor (NGF) (100 ng/ml) for 2 days. Cells were then fixed, and images were taken with a Zeiss LSM510 laser scanning confocal microscope. The insets are the 2× magnified images of the boxed areas. (B) Quantification of the total number of neurites, total length of neurites, and filopodium-like processes in a 20-μm neurite length. * indicates a significant difference compared with the respective control of GFP (P<0.05). (C) COS-1 cells were transfected with the control pcDNA3-GFP or pcDNA-M6Ab-GFP or pcDNA3-M6Ab-T166A-GFP plasmids. Cells were fixed, and images were taken with a Zeiss LSM510 laser scanning confocal microscope. Scale bars, 10 μm. |
|
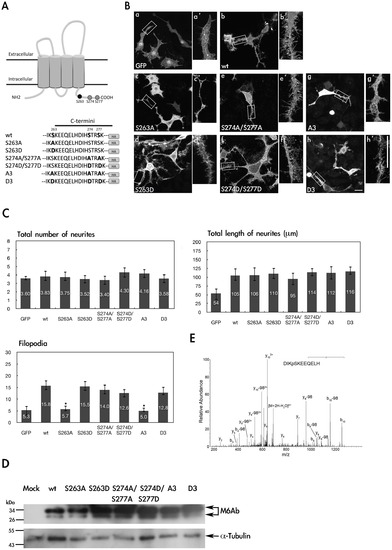
Phosphorylation of S263 is critical for regulation of filopodium formation in PC12 cells. (A) Partial amino acid sequences of the wide-type and mutant proteins of zebrafish M6Ab. (B) PC12 cells were transfected with pcDNA3-GFP-HA(a), pcDNA3-M6Ab-HA(b), pcDNA3-M6Ab(S263A)-HA(c), pcDNA3-M6Ab (S263D)-HA(d), pcDNA3-M6Ab(S274A/S277A)-HA(e), pcDNA3-M6Ab(S274D/S277D)-HA(f), pcDNA3-M6Ab(A3)-HA(g), or pcDNA3-M6Ab(D3)-HA(h) plasmids. Twenty-four hours after transfection, cells were treated with nerve growth factor (NGF) (100 ng/ml) for 2 days. Then transfected cells were fixed, and M6Ab-HA was detected using anti-HA antibodies for immunostaining. PC12 neurites are shown at a higher magnification (a2–h2). (C) Quantification of the total number of neurites, total length of neurites, and filopodium-like processes in a 20- · m neurite length. Results are expressed as the mean ± SD of at least 40~50 neurites. At least three independent experiments were analyzed. *Significant difference compared with the respective control of WT-M6Ab overexpression (P<0.05). (D) Cell lysates from different transfected cells as indicated were extracted and immunoblotted with an anti-HA antibody or anti-Tubulin antibody. (E) MS/MS spectrum on [M+2H]2+ (m/z 718.32) ion for the peptides DIKpSKEEQELH from WT M6Ab protein. The product ion y8 which carries a phosphate indicated that Serine 263 was phosphorylated. Residues bearing phosphate moieties are indicated with p. “b” and “y” ion series represent fragment ions containing the N- and C-termini of the peptide, respectively. Scale bars, 10 μm. |
|
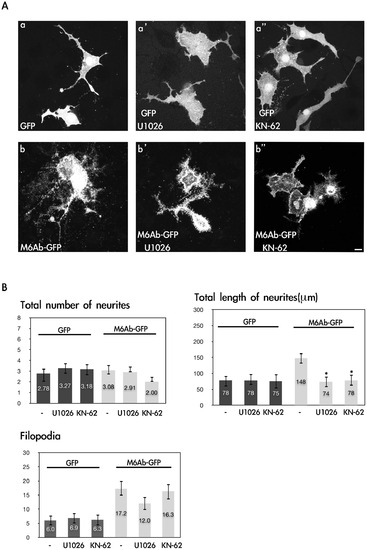
Inhibition of CaMKII and MEK1/2 reduces M6Ab early neurite outgrowth in nerve growth factor (NGF)-differentiated PC12 cells. (A) PC12 cells were transfected with the pcDNA3-M6Ab-GFP or pcDNA3-GFP plasmids. After transfection, PC12 cells were differentiated with 100 ng/ml NGF for 72 h in the presence of 10 μM KN-62 or 10 μM U1026 (Con; 0.1% DMSO). Cells were fixed, and images were taken with a Zeiss LSM510 laser scanning confocal microscope. (B) Quantification of the total number of neurites, total length of neurites, and filopodium-like processes in a 20-μm neurite length. Results are expressed as the mean ± SD of at least 40~50 neurites. At least three independent experiments were analyzed. *Significant difference compared with the respective control of WT M6Ab overexpression (P<0.05). Scale bars, 10 μm. |
|
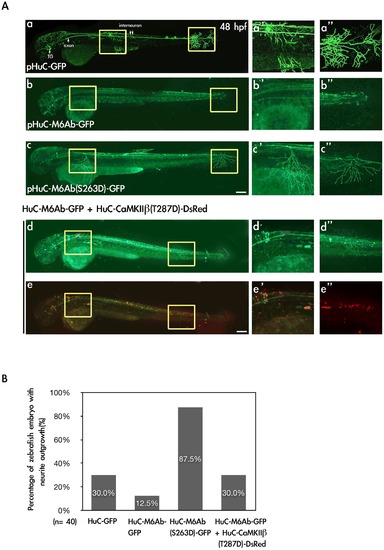
Expression of M6Ab-GFP and M6Ab(S263D)-GFP driven by a neuron-specific HuC promoter in zebrafish embryos. (A) To express the wild-type and S263D mutant proteins of zebrafish M6Ab under the control of a neuron-specific HuC promoter, plasmids pHuC-GFP, pHuC-M6Ab-GFP, pHuC-M6Ab(S263D)-GFP, and pHuC-M6Ab-GFP/pHuC-CaMKII · 1(T287D)-DsRed were individually injected into zebrafish embryos at the one-cell stage. Zebrafish embryos at 48 h post-fertilization (hpf) with GFP fluorescence were selected for the image analysis. Images were taken using a Zeiss LSM510 laser scanning confocal microscope. Merged images of red and green fluorescence are shown in (e, e2, and e3), while only green fluorescence images are shown in (a, a2, a3, b, b2, b3, c, c2, c3, d, d2 and d3). Higher magnification of two regions marked with yellow boxes in panel a-e is shown in panels a2–e2 and a3–e3, respectively. (B) Quantification of zebrafish numbers with significant neurite outgrowth at 48 hpf. Scale bars, 10 μm. |