- Title
-
Role of IGF signaling in catch-up growth and accelerated temporal development in zebrafish embryos in response to oxygen availability
- Authors
- Kamei, H., Ding, Y., Kajimura, S., Wells, M., Chiang, P., and Duan, C.
- Source
- Full text @ Development
|
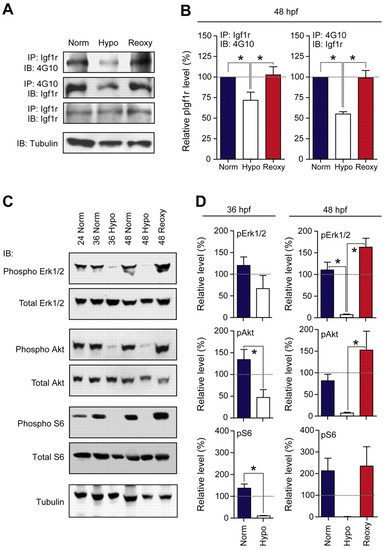
Hypoxia represses and re-oxygenation restores IGF signaling activities. (A) Representative results of biochemical analysis. Zebrafish embryos of the Norm, Hypo and Reoxy group shown in Fig. 1A were sampled at 48 hpf. Equal amounts of total protein were subjected to immunoprecipitation (IP) followed by immunoblotting (IB) using the indicated antibodies. (B) The phosphorylated/total Igf1r ratio. Data are mean + s.e.m., n=3-5; *, P<0.05. (C) Embryos of the Norm, Hypo and Reoxy groups shown in Fig. 1A were sampled at 24, 36 and 48 hpf, respectively. The samples were lysed and blotted using the indicated antibodies. (D) The phosphorylated/total protein ratio was quantified and values are expressed relative to that of the 24 hpf Norm group. Data are mean + s.e.m., n=3. *, P<0.05. |
|
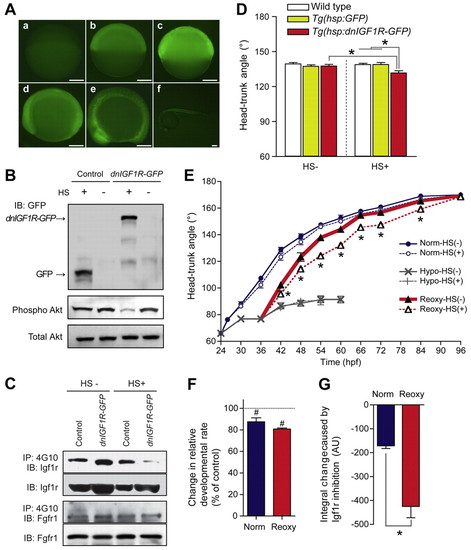
Temporally controlled blockade of Igf1r signaling inhibits catch-up growth. (A) Inducible expression of a dominant-negative form of Igf1r (dnIGF1R-GFP). Tg(hsp70:dnIGF1R-GFP) zebrafish embryos were exposed to a 1-hour heat shock (37°C) at 4 hpf and GFP signal was observed at 1 (b), 3 (c), 6 (d), 10 (e) and 20 hours (f) post heat shock. (a) No treatment control. Scale bars: 200 μm. (B) Tg(hsp70:dnIGF1R-GFP) and Tg(hsp70:GFP) embryos were exposed (or otherwise) to a 1-hour heat shock (HS) treatment at 4 hpf and raised to 24 hpf and analyzed by western blot using an anti-GFP antibody. The effect on Akt signaling was also examined and is shown in the lower panel. (C) Expression of dnIGF1R-GFP inhibited Igf1r signaling but had no effect on Fgfr1 signaling. Wild-type embryos and Tg(hsp70:dnIGF1R-GFP) embryos were exposed (or otherwise) to a 1-hour heat shock and analyzed by IP followed by IB analysis using the indicated antibodies. (D) Inhibition of Igf1r signaling decreases the HTA under normoxia. Tg(hsp70:dnIGF1R-GFP), Tg(hsp70:GFP) and wild-type embryos were exposed (or otherwise) to a 1-hour heat shock at 26 and 38 hpf and HTA was determined at 48 hpf. Data are mean + s.e.m., n=6-12; *, P<0.05. (E) Inhibition of IGF signaling blunts catch-up growth. Tg(hsp70:dnIGF1R-GFP) embryos were raised according to the experimental regime depicted in Fig. 1A. A subset of embryos from each treatment was subjected to a 1-hour heat shock every 12 hours starting at 26 hpf in the Norm group or 36 hpf in the Hypo and Reoxy groups. Owing to the developmental delay caused by hypoxia, the 36-hpf Hypo/Reoxy embryos were developmentally equivalent to those at 26 hpf in the Norm group. The HTA values were determined at the time points indicated. Data are mean ± s.e.m., n=9-17; *, P<0.05 between the Reoxy-HS(–) and Reoxy-HS(+) groups. (F,G) Inhibition of IGF signaling causes greater inhibition in the catch-up growth. Changes in relative developmental rate of Tg(hsp70:dnIGF1R-GFP) embryos were calculated in the Norm and Reoxy groups (F). The control (no heat shock) group was set as 100%. Data are mean + s.e.m., n=2; #, P<0.05 compared with the control. Cumulative change was calculated by area under curve (AUC) analysis (G). Values are shown as AUCHS(+) minus AUCHS(–). Data are mean + s.e.m., n=2; *, P<0.05. |
|
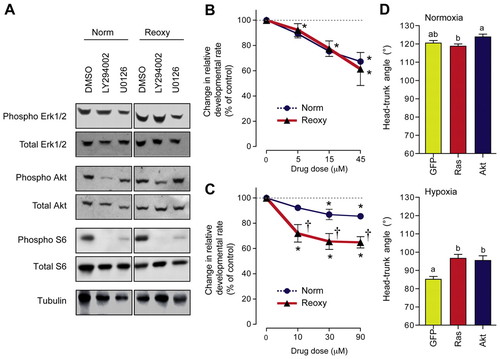
Distinct roles for the PI3K-Akt and Mek1/2-Erk1/2 signaling pathways in normal and catch-up growth. (A) Wild-type zebrafish embryos were raised following the experimental regime depicted in Fig. 1A. Embryos in the Norm group were treated with or without LY294002 (5 μM) or U0126 (10 μM) from 26 to 38 hpf. Embryos in the Reoxy group were treated from 36 to 48 hpf. Embryos were sampled in the middle of the experiment (4 hours post drug treatment) and the lysates were assayed for Erk1/2, Akt, S6 and Tubulin by immunoblotting. (B) Effect of LY294002 on normal and catch-up growth. Embryos were treated with LY294002 for 12 hours at the indicated doses. Data are mean ± s.e.m., n=3; *, P<0.05 compared with the no treatment control group. (C) Effect of U0126 on normal and catch-up growth. Embryos were treated with U0126 for 12 hours at the indicated doses. Data are mean ± s.e.m., n=3; *, P<0.05 compared with the control group; †, P<0.05 compared with the corresponding Norm group. (D) Forced expression of constitutively active Ras or Akt increases catch-up growth but has no effect on normal growth. Embryos injected with GFP, HA-RasV12 or myr-Akt mRNA (50 pg/embryo) were raised under normoxia or hypoxia until 36 hpf and the HTA determined. Data are mean + s.e.m., n=16-30. Different letters represent significant differences at P<0.05. |
|
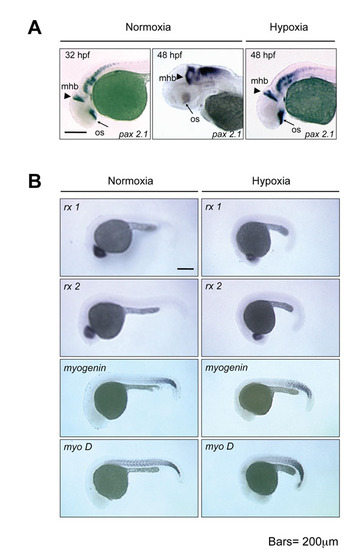
Hypoxia delays the timing of brain and somite morphogenesis but does not change patterning. (A) Whole-mount in situ hybridization of pax2a of embryos at 32 hpf in normoxia (stage-matched control), at 48 hpf in normoxia, and at 48 hpf in hypoxia. mbh, midbrain-hindbrain boundary; os, optic stalk. Scale bar: 200 μm. 12-hpf embryos were transferred to hypoxic or normoxic water, raised to the indicated stages, and subjected to whole-mount in situ hybridization. (B) 12-hpf embryos were transferred to hypoxic or normoxic water, raised to 24 hpf, and subjected to whole-mount in situ hybridization using the indicated markers. No pattern differences were found. |
|
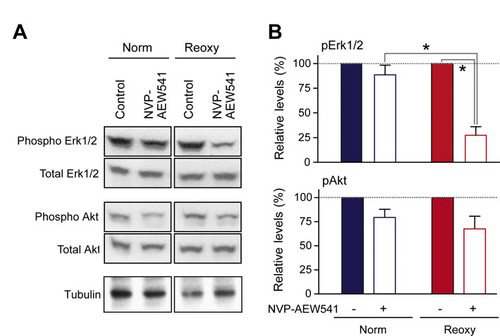
Inhibition of IGF signaling by NVP-AEW541. Embryos in the Norm group were treated with or without NVP-AEW541 and sampled at 48 hpf. Embryo lysates were blotted for phosphorylated and total Erk1/2 or Akt. Representative gels (A) and quantified ratios (B) are shown. Data are mean + s.e.m., n=3; *, P<0.05. |
|
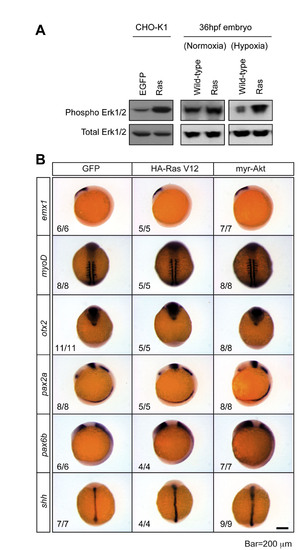
Effects of forced expression of constitutively active Ras and Akt. (A) Forced expression of a constitutively active Ras increases Erk1/2 activities in cultured CHO-K1 cells and zebrafish embryos. Western immunoblot analysis results of CHO-K1 cells transfected with HA-RasV12 or zebrafish embryos injected with GFP mRNA or HA-RasV12 mRNA (50 pg/embryo). (B) Expression of constitutively active Ras or Akt at this level does not alter patterning in zebrafish embryos. Expression of emx1 (forebrain), myoD (somite), otx2 (presumptive forebrain and midbrain), pax2a (optic stalk, mid-hindbrain boundary and hindbrain), pax6b (eye field and forebrain) and shh (notochord) in 12-hpf embryos injected with mRNA encoding GFP, HA-Ras V12 or myr-Akt. |