- Title
-
SSDP cofactors regulate neural patterning and differentiation of specific axonal projections
- Authors
- Zhong, Z., Ma, H., Taniguchi-Ishigaki, N., Nagarajan, L., Becker, C.G., Bach, I., and Becker, T.
- Source
- Full text @ Dev. Biol.
|
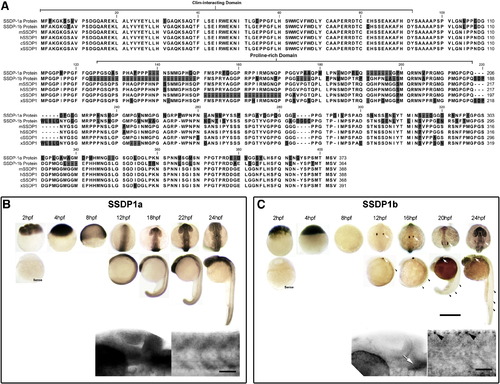
SSDP1a and SSDP1b protein sequences are conserved and mRNAs are expressed during embryonic development in zebrafish. A: The CLIM-interacting domain is most strongly conserved between zebrafish SSDP1s and the mouse (mSSDP1), human (hSSDP1), chick (cSSDP1) and Xenopus (xSSDP1) protein. B, C: Whole-mount in situ hybridization between 2 and 24 hpf indicates that SSDP1a is ubiquitously expressed with stronger expression in the head, whereas SSDP1b mRNA is most strongly expressed in trigeminal (white arrows) and Rohon–Beard (black arrowheads) neurons. Higher magnification pictures in lower row show lateral views of the head (left) and trunk (right). Upper scale bar in C = 500 μm for low magnification pictures; lower scale bar = 50 μm for high magnification pictures. |
|
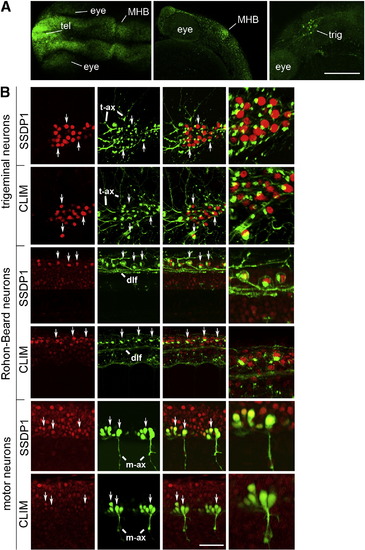
SSDP1 protein expression corresponds to in situ hybridization patterns and SSDP1 is co-expressed with CLIM protein in neuronal cell types. A: Dorsal (left) and lateral (middle, right) views of the head labeled with SSDP1 antibodies at 24 hpf are shown. Strong immunoreactivity is detectable in the telencephalon (tel), in the MHB and in the trigeminal ganglion (trig). The right panel is a selective z-stack of the epidermal region. Scale bar: 200 μm. B: Neuronal localization of SSDP1 and CLIM immunoreactivity (red) is revealed by co-labeling with an HNK-1 antibody (trigeminal and Rohon–Beard neurons in green) or by transgenic GFP expression in HB9:GFP transgenic embryos (motor neurons in green) at 24 hpf. The right outer column gives a higher magnification of double-labeled neurons. HNK-1 is strongly labeled on axonal membranes and Golgi apparatus next to nuclear labeling of SSDP1 and CLIM in trigeminal and Rohon–Beard neuron. In GFP expressing motor neurons, nuclear labeling is found inside the somata. Some double-labeled neurons are indicated by arrows. Trigeminal (t-ax) and motor axons (m-ax) as well as the dorsal longitudinal fascicle (dlf) are indicated for orientation. Scale bar: 50 μm for low and 100 μm for high magnification. |
|
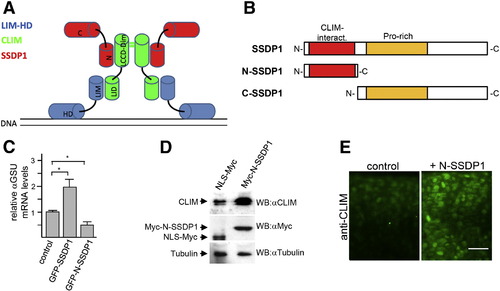
N-SSDP1 inhibits LIM-HD transcriptional complex in vitro and stabilizes CLIM in vivo. A: Model of transcriptional complexes consisting of LIM-HDs (blue), CLIM (green) and SSDP1 (red) on DNA. LIM domain (LIM); homeodomain (HD); LIM interaction domain (LID); Ldb/Chip conserved domain (LCCD); dimerization domain (dim); N- and C-terminal portions of SSDP1 (N and C, respectively) are indicated. B: N-SSDP1 contains the CLIM-interacting domain, but not the proline-rich domain. C-SSDP1 lacks the CLIM-interacting domain of SSDP1. C: Over-expression of full length SSDP1 in αT3 cells significantly increases transcriptional activity of LIM-HD factors in these cells whereas GFP-N-SSDP1 decreases it, as indicated by relative levels of the target αGSU mRNA measured by RT-qPCR. D: Western blot analysis of embryos (24 hpf) after over-expression of myc-tagged N-SSDP1 shows increased CLIM protein levels as compared to embryos injected with control mRNA for myc-tagged NLS. E: N-SSDP1 mRNA injection increases CLIM immunofluorescence in embryos, confirming Western Blot results. Lateral views of the trunk region of whole-mounted embryos (24 hpf) are shown. Scale bar = 25 μm. |
|
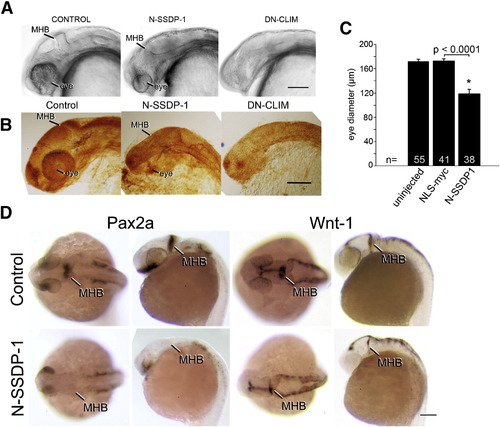
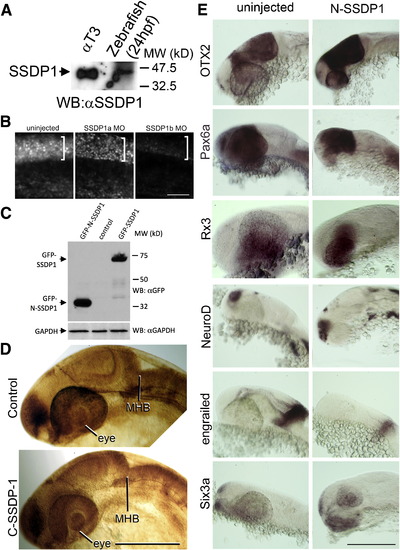
N-SSDP impairs eye and MHB development. A, B: Lateral views of the heads of living (A) or fixed, anti-tubulin immuno-labeled (B) 24 hpf embryos are shown. N-SSDP1 mRNA injection leads to reduced eye and MHB size compared to control embryos. DN-CLIM mRNA completely inhibits eye development and more strongly affects the MHB. C: Eye size is significantly reduced after N-SSDP1 mRNA over-expression compared to NLS-myc control mRNA over-expression. D: Dorsal (left columns) and lateral views (right columns) of whole-mounted 24 hpf embryos reveal loss of Pax2a and reduction of Wnt-1 mRNA expression in the MHB after N-SSDP1 mRNA over-expression. Scale bars = 200 μm (A, B and D). |
|
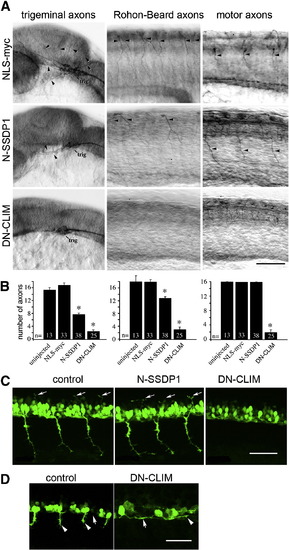
Phenotypes induced by N-SSDP over-expression partially overlap with those induced by DN-CLIM over-expression. A: Lateral views of anti-tubulin immuno-labeled whole-mounted 24 hpf embryos are shown. Injection of N-SSDP1 mRNA leads to loss of peripheral axons of trigeminal (trig) and Rohon–Beard neurons, but not of ventral motor axons (NLS-myc = control mRNA). DN-CLIM mRNA injections affect all three types of axons. Arrows indicate axons. B: Quantification confirms significant loss of peripheral sensory but not motor axons upon N-SSDP1 over-expression, whereas DN-CLIM over-expression affects also motor axons. C: DN-CLIM, but not N-SSDP1 over-expression inhibits growth of dorsal MiP axons (arrows), as shown in lateral trunk views of HB9:GFP transgenic embryos at 28 hpf. D: Higher magnification of the ventral border of the caudal spinal cord (arrows) at 24 hpf shows that HB9:GFP+ motor neurons grow axons (arrowheads) ventrally out of the spinal cord in controls. In DN-CLIM mRNA injected embryos axons grow along the ventral border of the spinal cord. Scale bars = 50 μm. |
|
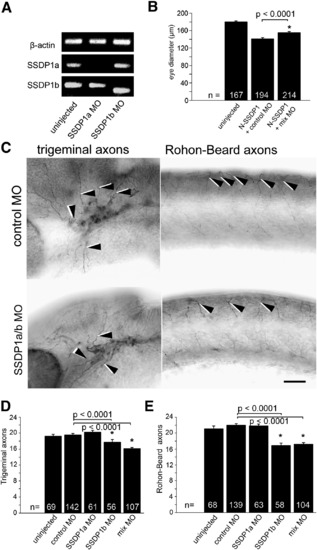
Knock down of SSDP1b, but not SSDP1a partially inhibits growth of peripheral axons of trigeminal and Rohon–Beard neurons. A: Morpholino knock down reduces levels of correctly spliced SSDP1a and SSDP1b to levels that are undetectable by PCR. An ectopic band is amplified after SSDP1b knock down, which contains premature stop codons and, therefore, cannot lead to functional protein. Beta-actin was used to equalize cDNA concentrations. B: Morpholinos to SSDP1 partially rescue reduced eye size induced by N-SSDP1 over-expression, suggesting specificity of the morpholinos. C: Lateral views of anti-tubulin immuno-labeled whole-mounted 24 hpf embryos injected with morpholinos against SSDP1a/b are shown. Arrowheads point to trigeminal and Rohon–Beard axons, respectively. Scale bar = 50 μm. D, E: Quantifications indicate significant loss of peripheral axons of trigeminal and Rohon–Beard neurons after morpholino knock down of SSDP1b. PHENOTYPE:
|
|
A: Western blot analysis indicates that the SSDP1 antibody detects a band of the same molecular weight in protein extracts of αT3 cells and zebrafish embryos, suggesting specific reactivity of the antibody in zebrafish embryos. B: Immunodetectability of SSDP1 is slightly reduced by SSDP1a and strongly reduced by SSDP1b morpholino injection, indicating that the morpholino predominantly recognizes SSDP1b. Lateral trunk views of whole-mounted embryos are shown. The strongly immunoreactive spinal cord level is indicated by brackets. C: Western blot analysis indicates that GFP-tagged N-SSDP1 and full length SSDP1 can be expressed in αT3 cells. D: Lateral views of anti-tubulin labeled embryos at 24 hpf are shown. Over-expression of C-SSDP1, which lacks the CLIM-interacting domain, does not induce phenotypes in eye or MHB. E: Lateral views of the heads of 24 hpf embryos after in situ hybridization are shown. The indicated markers retain their general expression patterns when N-SSDP1 is over-expressed. Scale bar in B = 50 μm; bars in D,E = 200 μm. |
|
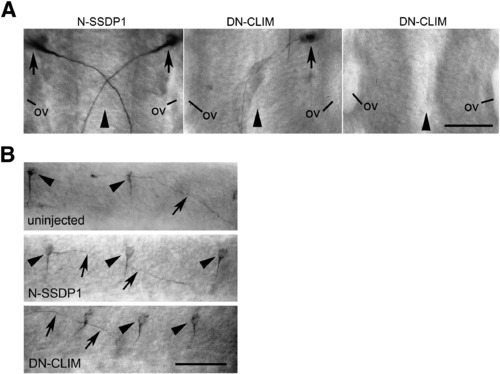
DN-CLIM but not N-SSDP1 over-expression induces patterning defects specifically in the hindbrain. A: Dorsal views of the hindbrain (rostral is up) of 24 hpf embryos immuno-labeled with the 3A10 antibody are shown. Development of Mauthner neurons (arrows) and crossing of their axons at the brain midline (arrowhead) is not affected by N-SSDP1 over-expression. In contrast, DN-CLIM over-expression leads to complete or partial loss of Mauthner neurons. Note that in those cases in which only one Mauthner neuron is present its axonal trajectory is not altered (ov = otic vesicle). B: Lateral views of the spinal cord show that 3A10 immuno-labeled CoPA neurons (arrowheads) and their commissural axons (arrows) are not affected by N-SSDP1 or DN-CLIM over-expression. Scale bars = 50 μm. |
|
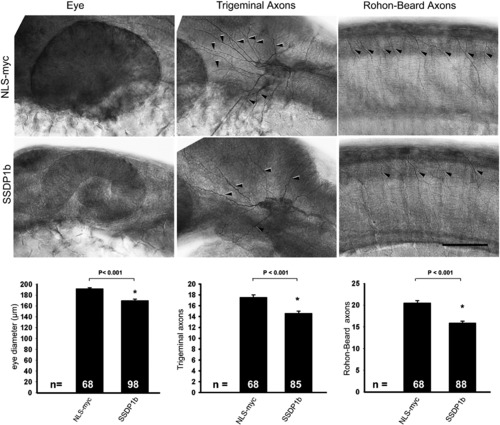
Full length zebrafish SSDP1b over-expression has similar effects to N-SSDP1. Lateral views of anti-tubulin labeled whole-mounted 24 hpf embryos are shown. Arrowheads indicate axons. Eye size as well as axon numbers of trigeminal and Rohon–Beard neurons are significantly reduced by SSDP1b over-expression compared to NLS-myc control. Scale bar = 50 μm. |
|
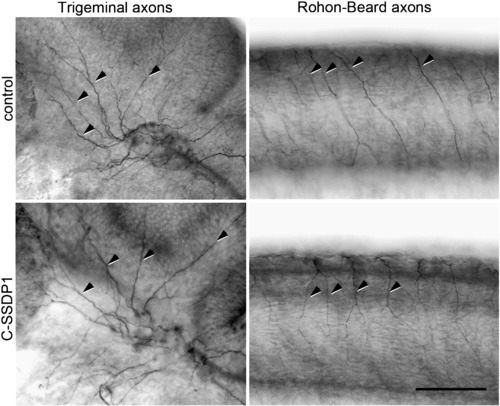
C-SSDP1 over-expression does not affect peripheral trigeminal or Rohon–Beard axons. Lateral views of head (for trigeminal axons) and trunk (for Rohon–Beard axons) are shown. Some of the axons are indicated by arrows. Scale bar = 100 μm. |
Reprinted from Developmental Biology, 349(2), Zhong, Z., Ma, H., Taniguchi-Ishigaki, N., Nagarajan, L., Becker, C.G., Bach, I., and Becker, T., SSDP cofactors regulate neural patterning and differentiation of specific axonal projections, 213-224, Copyright (2011) with permission from Elsevier. Full text @ Dev. Biol.