- Title
-
Differential requirement for BMP signaling in atrial and ventricular lineages establishes cardiac chamber proportionality
- Authors
- Marques, S.R., and Yelon, D.
- Source
- Full text @ Dev. Biol.
|
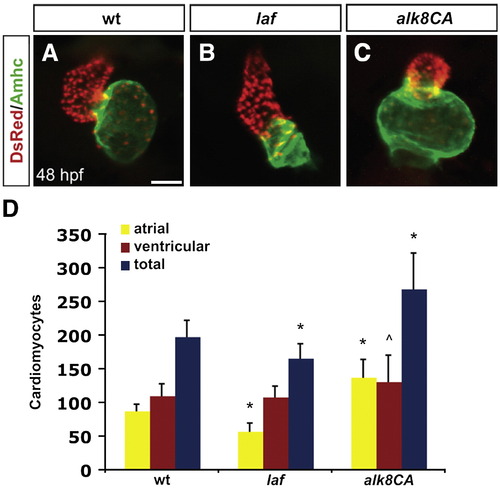
Alk8 function regulates chamber proportionality. (A–C) Frontal views of hearts from wild-type (A), laf mutant (B) and alk8CA mRNA-injected (C) embryos at 48 h.p.f. Scale bar represents 50 μm. Immunofluorescence detects DsRed (red) in all cardiomyocyte nuclei and atrial myosin heavy chain (Amhc; green) in atrial cells. (A) In a wild-type heart, the ventricle (red) and atrium (green) exhibit typical looping and expansion. (B) In a laf mutant heart, the chambers are unlooped and dysmorphic. (C) In alk8CA mRNA-injected embryos displaying a v2–v3 class of ventralized body morphology (Kishimoto et al., 1997), the hearts are enlarged with a particularly striking expansion of the atrium. (D) Quantification of cardiomyocyte number in wild-type, laf mutant and alk8CA mRNA-injected embryos at 48 h.p.f. Graph indicates the mean and standard deviation for each data set; see also Table S1. Statistically significant differences from wild-type are indicated by asterisks (p < 0.005) or carets (p < 0.05). n = 29 for wild-type, n = 19 for laf mutants, and n = 10 for alk8CA mRNA-injected embryos. laf mutants and alk8CA mRNA-injected embryos have disproportionately small or large atrial chambers, respectively. |
|
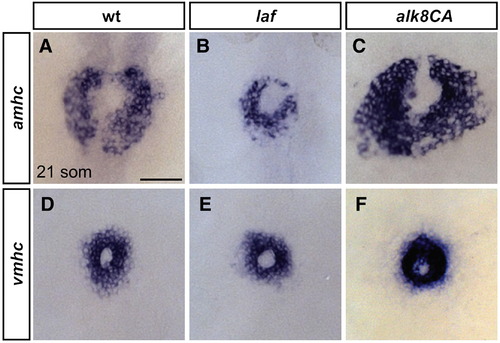
Alk8 function impacts atrial cell number prior to heart tube formation. (A–F) In situ hybridization depicts expression of amhc (A–C) and vmhc (D–F) at the 21-somite stage; dorsal views, anterior to the top. Scale bar represents 50 μm. (A) In wild-type embryos, amhc is expressed in a ring of atrial cardiomyocytes just prior to heart tube extension, whereas in laf mutant embryos (B), the population of amhc-expressing cells is clearly reduced (n = 14/15). (C) In alk8CA mRNA-injected embryos displaying a v2–v3 ventralized body morphology, the population of amhc-expressing cells is clearly increased (n = 20/23). (D) vmhc is expressed in a ring of ventricular cardiomyocytes, located interior to the ring of atrial cardiomyocytes. The population of vmhc-expressing cells is comparable to wild-type in (E) laf mutants (n = 14/14) and seems slightly increased in (F) alk8CA mRNA-injected embryos (n = 22/22). EXPRESSION / LABELING:
|
|
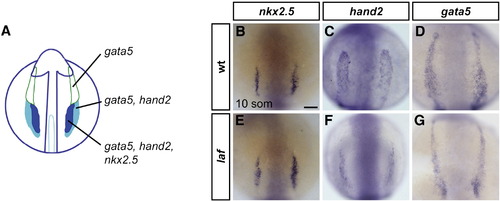
laf mutants exhibit lateral plate mesoderm deficiencies. (A) Schematic representation of gene expression patterns in the ALPM in an embryo at the 10-somite stage (Schoenebeck et al., 2007); dorsal view, anterior to the top. gata5 is expressed throughout the two bilateral strips of ALPM. hand2 overlaps with gata5 in the posterior regions of the ALPM, which correspond to the heart fields. nkx2.5 overlaps with hand2 in the medial portions of the heart fields. (B–G) In situ hybridization depicts expression of nkx2.5, hand2 and gata5 at the 10-somite stage; dorsal views, anterior to the top. Scale bar represents 50 μm. (B–D) are wild-type embryos and (E–G) are laf mutant siblings. (B, E) nkx2.5 expression is indistinguishable in wild-type and laf mutant embryos (n = 15/15). (C, D, F, G) laf mutants display narrowed domains of hand2 (n = 18/20) and gata5 (n = 17/20) expression, revealing a defect in the width of the cardiac ALPM. hand2 and gata5 expression levels vary from normal to reduced in laf mutants, which also exhibit reduced expression of other cardiac progenitor markers, including gata4, nkx2.7, and tbx5 (SRM and DY, unpublished data). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Inhibition of BMP signaling during gastrulation results in decreased cardiomyocyte numbers. (A–C) Lateral views of embryos exposed to dorsomorphin from 50% epiboly (B) or tailbud (C) stages until 48 h.p.f.; all images are at the same magnification. (B) Embryos treated with dorsomorphin from 50% epiboly onward lack the ventral tail fin (arrowheads), phenocopying the laf mutant body morphology. (C) Embryos treated with dorsomorphin from tailbud stage onward display mild abnormalities of the ventral tail fin (arrowheads), which is smaller in comparison to control embryos (A, arrowheads) treated with the same concentration of DMSO. (D) Quantification of cardiomyocyte number at 48 h.p.f. following dorsomorphin treatment. Graph as in Fig. 1; see also Table S2. n = 10 for control DMSO treatment, n = 10 for 50% epiboly treatment, n = 11 for tailbud treatment. (E) Quantification of cardiomyocyte number at 48 h.p.f., following heat shock of embryos carrying the transgene Tg(hsp701:dnBmpr-GFP). Control embryos were non-transgenic siblings, and heat shocks were performed at 65% epiboly, 75% epiboly, and the 8-somite stage. Graph as in Fig. 1; see also Table S3. n = 18 for non-transgenic embryos, n = 14 for heat shock at 65% epiboly, n = 17 for heat shock at 75% epiboly, and n = 17 for heat shock at the 8-somite stage. Statistically significant differences from wild-type are indicated by asterisks (p < 0.005) or carets (p < 0.05). Differences observed between the effects of dorsomorphin exposure and heat shock may be explained by differences in the strength of inhibition provided by each method. PHENOTYPE:
|
|
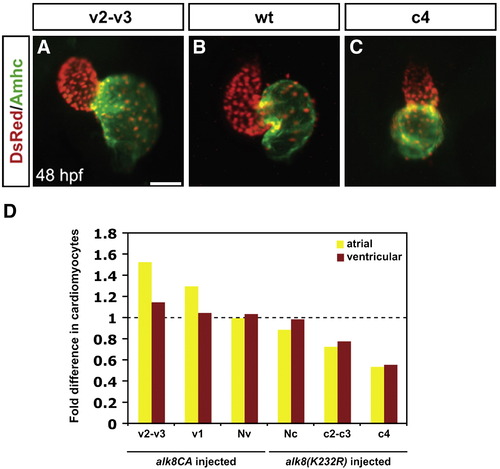
Levels of BMP signaling affect cardiomyocyte number and proportion in a dose-dependent manner. (A–C) Frontal views of hearts at 48 h.p.f.; immunofluorescence as in Fig. 1. Scale bar represents 50 μm. Hearts depict examples from (A) an alk8CA mRNA-injected embryo displaying a v2–v3 class of severely ventralized body morphology (Kishimoto et al., 1997), (B) a wild-type embryo, and (C) an alk8(K232R) mRNA-injected embryo displaying a c4 class of severely dorsalized body morphology (Mullins et al., 1996). (D) Quantification of cardiomyocytes at 48 h.p.f., following injection of alk8CA or alk8(K232R) mRNA. Injected embryos were separated into previously described phenotypic classes based on body morphology. Injection of alk8CA mRNA causes a range of ventralization classes (v2–v3, v1, and Nv, meaning normal morphology) (Kishimoto et al., 1997). Injection of alk8(K232R) mRNA causes a range of dorsalization classes (Nc, meaning normal morphology, c2–c3 and c4) (Mullins et al., 1996). Graph indicates fold difference in mean values for each phenotypic class relative to wild-type embryos. |
|
BMP signaling is required cell autonomously for cardiomyocyte formation. (A, B) Example of transplantation results; lateral views, ventral to the top, anterior to the left. (A) Wild-type donor-derived cells expressing Tg(cmlc2:egfp) in wild-type host myocardium at 48 h.p.f. Scale bars represent 50 μm. (B) Overlay of bright-field and fluorescent images revealing that the donor-derived cells are located in the outer curvature of the ventricle. (C) Table of transplantation results indicating the frequency of myocardial contribution of wild-type donor cells or smad5 morphant donor cells in wild-type host embryos. Cells from smad5 morphant donors contribute significantly less often to myocardium than do cells from wild-type donors; asterisk indicates p < 0.05. In one example of smad5 morphant cardiomyocyte contributions, donor-derived cells were found in the ventricle; in the other example, donor-derived cells were found in both the atrium and the ventricle. (D, E) Detection of wild-type and smad5 morphant donor-derived cells in the skeletal muscle of wild-type host embryos at 48 h.p.f. by anti-fluorescein immunohistochemistry. Scale bars represents 50 μm. Lateral views, dorsal to the top, anterior to the left. (F) Table of transplantation results indicating the equivalent frequencies of skeletal muscle contribution of wild-type donor cells or smad5 morphant donor cells in wild-type host embryos. Differences between the frequency of wild-type contribution to myocardium and skeletal muscle are likely due to differences in organ field size. Since the skeletal muscle field is larger than the heart field (Kimmel et al., 1990), wild-type donor cells contribute to skeletal muscle more frequently. |
Reprinted from Developmental Biology, 328(2), Marques, S.R., and Yelon, D., Differential requirement for BMP signaling in atrial and ventricular lineages establishes cardiac chamber proportionality, 472-482, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.