- Title
-
Mifepristone-inducible LexPR system to drive and control gene expression in transgenic zebrafish
- Authors
- Emelyanov, A., and Parinov, S.
- Source
- Full text @ Dev. Biol.
|
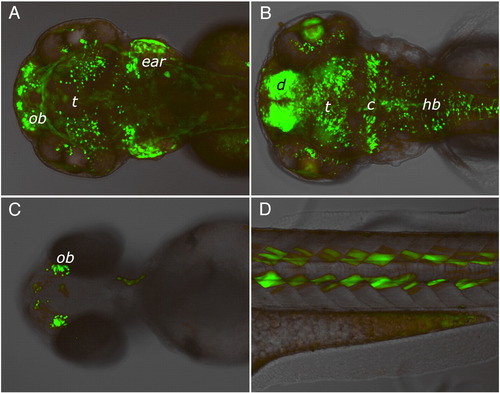
EGFP expression patterns in four independent LexPR driver-reporter lines showing different enhancer-trap events. Expression of EGFP reporter was induced in transgenic F2 embryos by adding mifepristone to the egg water at 1 μM final concentration at 24 hour post-fertilization onwards. Images were captured at 96 hour post-fertilization. No EGFP fluorescence was detected in the control populations of fish that were not treated with mifepristone (not shown). (A) Strong expression in the cell layer at the bottom of the ear (possibly sensory patches), at the olfactory bulb region (ob) and in discrete cells of midbrain (head, dorsal view). (B) Strong expression in the diencephalon (d) and cerebellum (c), lens and in discrete cells of the tectum (t) and hindbrain (hb) (head, dorsal view). (C) Expression in the olfactory bulbs (head, ventral view). (D) Specific expression in a subset of muscle cells in the somites (lateral view above yolk extension). |
|
Rate of mifepristone-induced activation of transcription. Whole mount RNA in situ hybridizations with the LexPR- (A) and EGFP-specific (B–G) antisense RNA probes. The transgenic driver-reporter line used here normally expresses EGFP in a subset of tissues shown in Fig. 2A. (A) Expression pattern of the LexPR transactivator RNA at 48 hpf. The strongest expression is found in the cell layer at the bottom of the otic capsule (possibly sensory patches) and at the olfactory bulb region. The diffused expression in the dorsal brain appears quite strong on the lateral view due to specimen thickness; the expression is actually weak as seen on the dorsal view. The 72 hpf LexPR expression pattern is similar (image not shown). (C–G) EGFP reporter transcript 1 (C), 3 (D), 6 (E), 9 (F) and 24 (G) hours after induction with 1 μM mifepristone (RU486) at 48 hpf stage onwards. No background EGFP transcript was observed without mifepristone (B). |
|
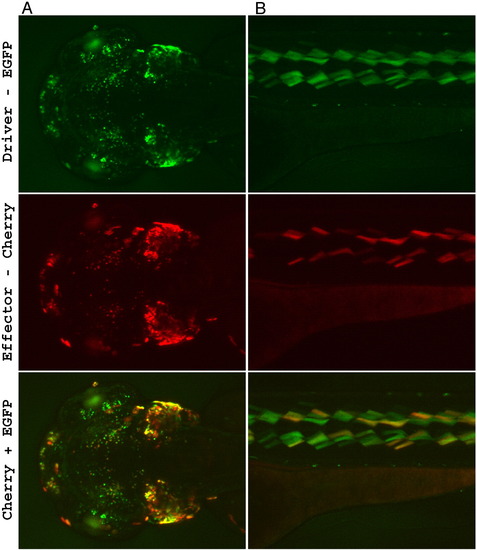
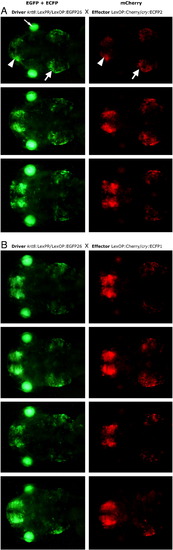
Driver-effector trans-activation. Two independent driver lines with different LexPR transactivator expression patterns were studied: (A) — represents the driver line shown in Fig. 2A, (B) — the driver line shown in Fig. 2D. Each driver line was crossed with a transgenic effector line (different effector lines were used in panels A and B), which carried mCherry gene under control of the LexOP. The hybrid F3 embryos that were double transgenic for the driver and effector cassettes were treated with 1 μM mifepristone from 24 hpf onwards. Bottom panel is a combined image showing both reporters EGFP and mCherry: green indicates excess of EGFP fluorescence, red — excess of mCherry fluorescence, yellow — similar levels of mCherry and EGFP fluorescence (notice that these colors do not constitute true molar ratios between the reporters). Although, most positive cells express both reporters, there are cells that yield brighter mCherry fluorescence than the EGFP and vice-versa. mCherry fluorescence is not detected in the effector lines containing only the effector cassette upon treatment with mifepristone. No background EGFP/mCherry fluorescence was detected in the control populations of double transgenic fish that were not treated with mifepristone (not shown). Images were captured at 96 hour post-fertilization. (A) Strong expression is in the cell layer at the bottom of the ear (possibly sensory patches), at the olfactory bulb region and in various neurons (head, dorsal view). Notice the autofluorescence from the iridophores in the eye that is visible only through the EGFP filter set. (B) Expression is detected in the specific subtype of muscle cells (lateral view). Two lines of dots show autofluorescence from the iridophores. |
|
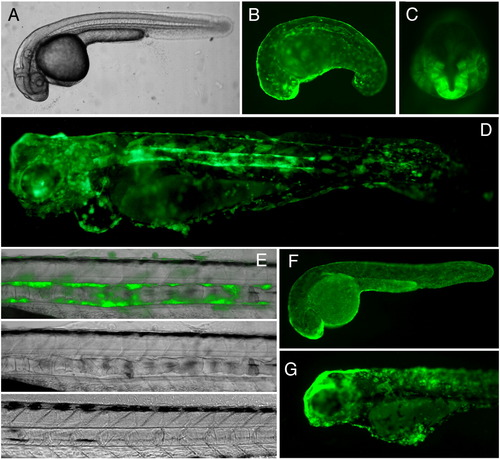
Inducible expression of EGFP-KrasV12 in transgenic zebrafish. Transgenic zebrafish line harboring insertion of the binary driver construct Ds(krt8:LPR-LOP:EGFP-KrasV12) containing EGFP-KrasV12 under control of the LexOP, F2 (A–E and G). (A) 24 hpf, no induction. (B, C) 24 hpf, 1 μM mifepristone treatment from 10 hpf onwards. GFP fluorescence can be observed in the skin epithelia, the notochord sheath (lateral view, B), in the forebrain (ventral view, C), all of these organs are abnormal. (D) 4 dpf, 1 μM mifepristone treatment 10 hpf onwards, lateral view. Notochord, brain and cranial skeleton are severally affected by the EGFP-KrasV12 expression. Skin epithelial cells lost epithelial cell shape, became globular, formed clusters. (E) Transformation of the sheath cells surrounding the notochord. The top and the middle panel shows the notochord of 4 dpf fish treated with 1 μM mifepristone from 10 hpf onwards. Bottom panel shows notochord of the untreated control. (F) Transgenic control line that harbors the driver-reporter construct pDs(krt8:LPR-LOP:EGFP) containing EGFP gene under control of the LexOP. 24 dpf, 1 μM mifepristone treatment starting at 10 hpf, showing EGFP expression in the skin epithelia. The skin cells have flat epithelial shape and uniformly cover the entire body. (G) 4 dpf. Expression of EGFP-KrasV12 was induced with 1 μM mifepristone treatment from 1 dpf onwards. Due to later induction, there is much less developmental abnormalities, but the cells of skin epithelia cells acquired rounded shape and formed clusters instead of a continuous layer. |
|
Testing the LexPR transcription control using RT-PCR. RT-PCR was performed on RNA extracted from transgenic F2 embryos carrying the driver-reporter construct using two pairs of primers to detect EGFP (top panel) and LexPR transactivator (bottom panel) RNA products. Embryos (36 hpf) were treated with 1μM mifepristone for 12 hours (48hpf). After that, the embryos were rinsed and transferred into larger tanks without mifepristone. Six mRNA samples were prepared and tested: 1) before induction (36 hpf); 2) after 1-hour induction; 3) after 12-hours induction; 4) 1 day after mifepristone withdrawal; 5) 5 days after mifepristone withdrawal; 6) wild type - negative control. The PCR-conditions (number of cycles) were optimized for detection of low quantities of RNA target, not for quantitative comparison between samples. Thus, there is in no visible product downregulation at the lane 5 (5 days post withdrawal). |
|
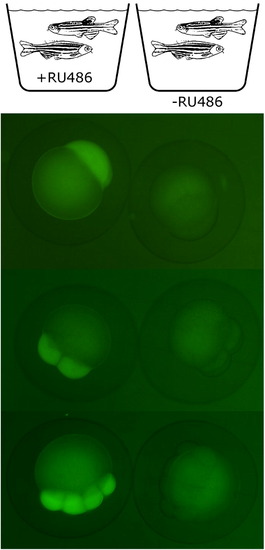
Control of maternal expression in the transgenic fish that harbor an enhancer-trap insertion of krt8:LexPR/LexOP:EGFP construct driving maternal expression of LexPR transactivator. A transgenic F2 female and a WT male were placed in the crossing tank containing 1 μM mifepristone approximately 12 h before the fertilization. All the F3 embryos showed maternal EGFP expression (left panel). In contrast, the offspring of the transgenic females untreated with mifepristone did not express EGFP at the early stages (right panel). The embryos are shown at 1, 4 and 8-cell stages.[GR6] |
|
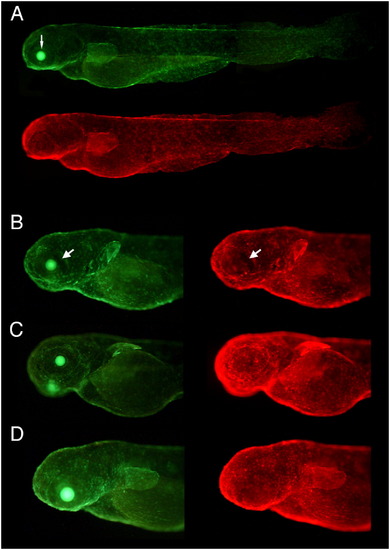
Uniformity and consistency of expression in double transgenic fish that harbor a driver-reporter Ds(promoter:LexPR-LexOP:EGFP) and an effector Ds(cry:ECFP-LOP:mCherry) constructs. Effector construct contains permanent selection marker crystallin:ECFP which produces cyan expression in the lens (thin arrow) visible through the GFP filter set but not through the mCherry filter set. I. Two different driver-reporter lines with the same promoter crossed to the same effector line. The gfap:LexPR-LexOP:EGFP driver-reporter construct harbors the LexPR transactivator sequence under control of the gfap (glial fibrillary acidic protein) promoter that results in expression of the EGFP reporter in the central nervous system. A (lateral view) and C (dorsal view) show several F2 fish from the cross between the driver line #4 female and the effector line #1 male. B is a progeny of the independent Driver line #6 crossed with the same Effector line #1. There is strong expression in the forebrain (arrowhead) and in the a few segments of the hindbrain (large arrows). |
|
II. The same driver line crossed to two independent effector lines. The driver line contains an enhancer-trap insertion of krt8:LexPR-LexOP:EGFP construct that results in expression of the EGFP reporter in the forebrain (arrowhead) and in the otic capsule (large arrow). A shows three different F2 fish randomly taken from the cross between the Driver line #26 female and the Effector line #2 male. B — is a progeny of the same Driver line #26 crossed with the different Effector line #1. |
|
III. A–D. Mosaic reporter expression in the skin epithelia. A–D. The krt8:LexPR-LexOP:EGFP driver construct contains a promoter of the keratin8 (krt8) gene that drives expression of LexPR transactivator in the single layer of epithelial skin cells of the same type. Shown are four F2 fish from the same parents. Although most epithelial cells express both reporters, there is visible variation of intensity between cells. There are also patches of cells that express very weak level of both reporters (large arrow), that may reflect positional effects at the driver insertion site or silencing of the LexPR gene, the difference in drug permeability among the cells of the same type or other difference between these cells (age, stress, etc) at the time of induction. A. Assembled image of the whole fish produced by stitching several overlapped exposures.[GR7] |
|
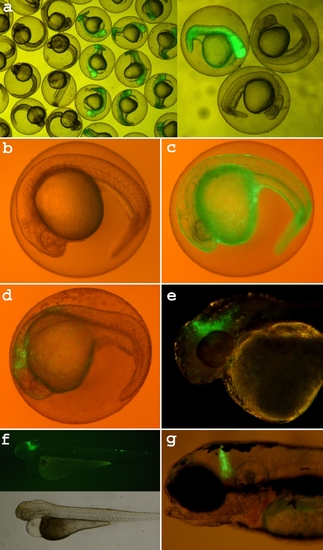
Side effects caused by strong early expression of Gal4/UAS-based expression systems. a) Strong expression of non-inducible Gal4/UAS system in true transgenic fish at early developmental stages (less than 24 hpf) caused serious abnormalities that became evident later in development. d-f) Mifepristone-induced Gal4/UAS expression caused less pronounced developmental abnormalities compared to the non-inducible system when induced before 24hpf. In some transgenic fish the phenotypes were subtle (g) and some survived to maturity. Induction after 24hpf did not cause serious effects although sometimes affected individual cell morphology. For both non-inducible and inducible Gal4/UAS-based systems severity of the phenotypes strongly correlated with the intensity of reporter expression. |
Reprinted from Developmental Biology, 320(1), Emelyanov, A., and Parinov, S., Mifepristone-inducible LexPR system to drive and control gene expression in transgenic zebrafish, 113-121, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.