- Title
-
Subfunctionalization of Duplicated Zebrafish pax6 Genes by cis-Regulatory Divergence
- Authors
- Kleinjan, D.A., Bancewicz, R.M., Gautier, P., Dahm, R., Schonthaler, H.B., Damante, G., Seawright, A., Hever, A.M., Yeyati, P.L., van Heyningen, V., and Coutinho, P.
- Source
- Full text @ PLoS Genet.
|
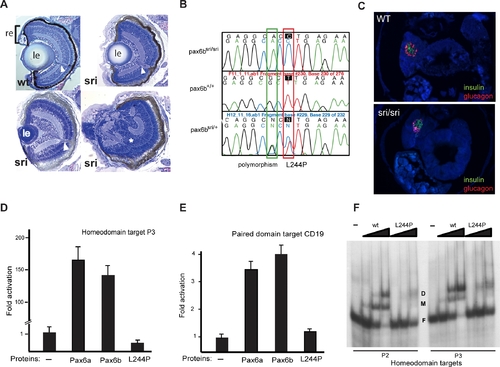
Characterisation of the sunrise (sri) Mutant PHENOTYPE:
|
|
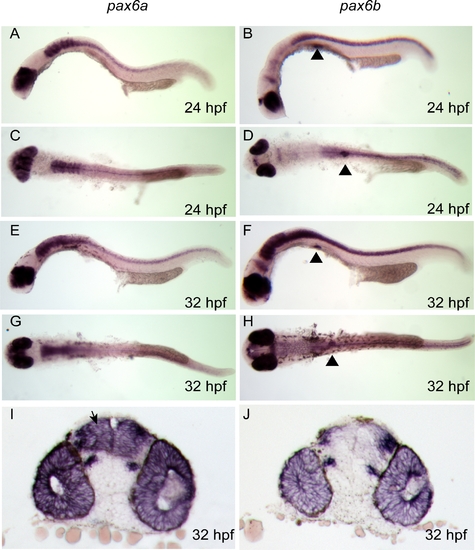
pax6a and pax6b Expression Analysis by Wholemount RNA in situ Hybridisation during Early Zebrafish Development |
|
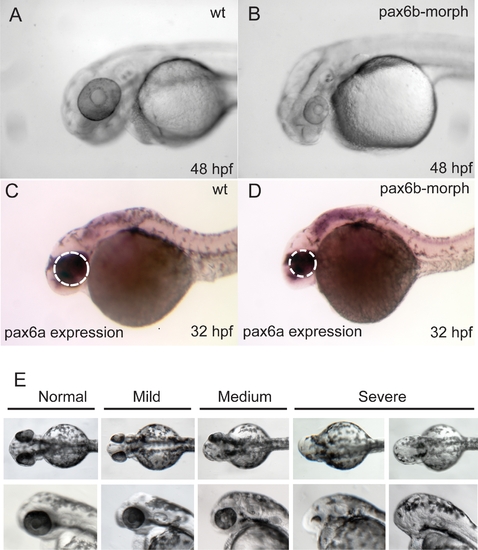
Analysis of pax6b Function in Zebrafish, with the Aim of Validating the Effect of the pax6b L244P Missense Mutation in sri Fish |
|
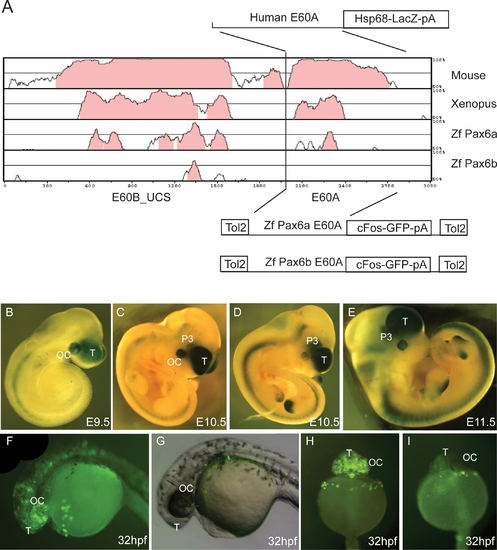
Enhancer Capacity of the E60A Conserved Element Assessed in Transgenic Mice and Zebrafish. |
|
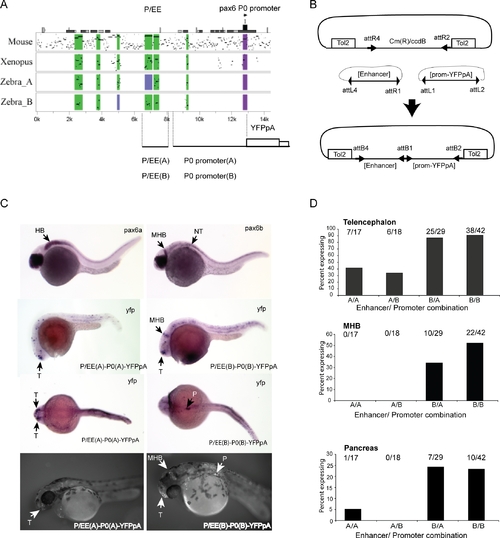
Transgenic Analysis of the Zebrafish P/EE Enhancers from the pax6a and pax6b Loci in Combination with the pax6 P0 Promoters EXPRESSION / LABELING:
|
|
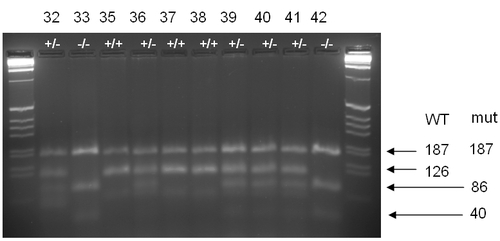
Genotyping of sri mutant and wild type larvae |
|
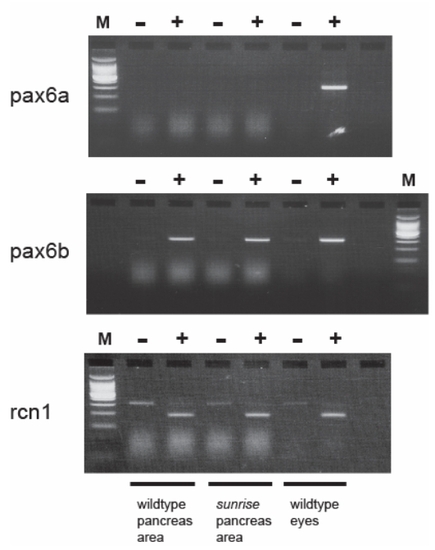
RT-PCR Analysis Shows That Only pax6b Is Expressed in the Pancreas of Both Wild Type and sri/sri Homozygous Adult Fish. In Contrast, Both pax6a and pax6b Are Expressed in Adult Eye. EXPRESSION / LABELING:
|

Unillustrated author statements EXPRESSION / LABELING:
|