- Title
-
Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer's vesicle in zebrafish
- Authors
- Amack, J.D., Wang, X., and Yost, H.J.
- Source
- Full text @ Dev. Biol.
|
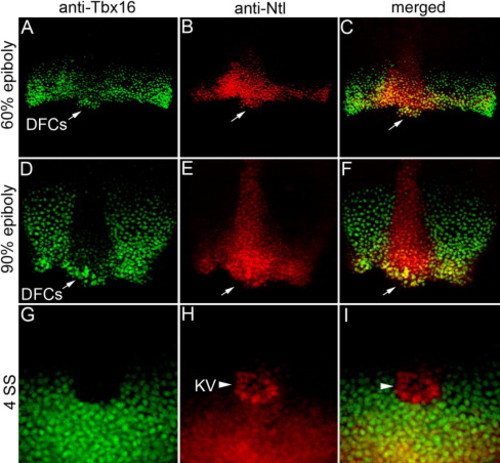
Ntl and Tbx16 proteins are co-expressed in DFCs. (A–I) Confocal microscopy of double fluorescent immunostaining in wild-type embryos using anti-Tbx16 (green) and anti-Ntl (red) antibodies. Ntl and Tbx16 co-localized in the nuclei of margin cells and dorsal forerunner cells (DFCs; arrows in panels A–F) at 60% epiboly (A–C) and 90% epiboly (D–F) stages. At 4 SS, Tbx16 expression (G) was detected in tailbud cells surrounding Kupffer's vesicle (KV), but not in KV cells marked by Ntl expression (arrow head in panels H, I). EXPRESSION / LABELING:
|
|
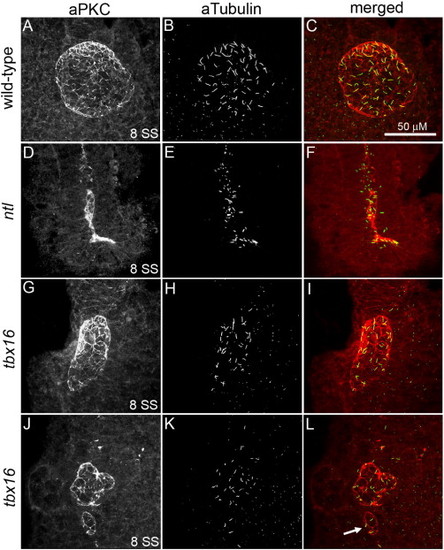
KV morphological defects are different in ntl and tbx16 mutants. (A–L) Confocal microscopy of double fluorescent immunostaining of aPKC (red) and aTubulin (green) in KV cells at 8 SS. In wild-type embryos (A–C), aPKC marked the apical surface of Kupffer's vesicle (KV) cells that line the spherical KV lumen and aTubulin labeled cilia protruding into the luminal space. aPKC+/aTubulin+ KV cells were present in both ntl (D–F) and tbx16 (G–L) mutant embryos. ntl mutants consistently lacked a KV lumen (D–F). In contrast, a KV lumen formed in the majority of tbx16 mutant embryos, but was reduced in size, dysmorphic (G–L) and often accompanied by ‘mini-vesicles’ (arrow in panel L). All confocal micrographs are at the same scale (see scale bar in panel C). PHENOTYPE:
|
|
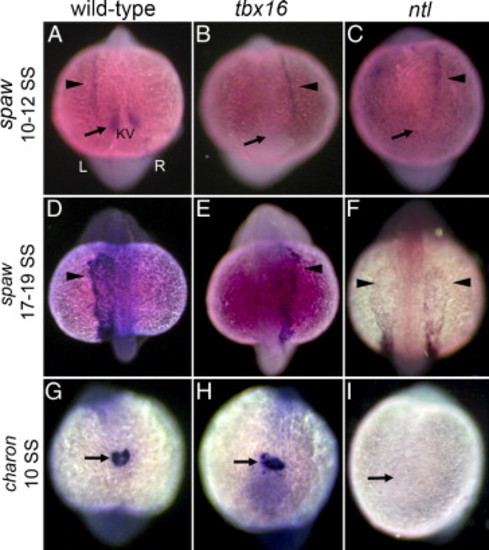
Asymmetric gene expression is initiated randomly in ntl and tbx16 mutants. (A–C) At 10–12 SS spaw is normally expressed in a symmetric ‘perinodal’ domain flanking KV (arrow in panel A) and is just being initiated asymmetrically in left LPM (arrowhead in panel A). All views are dorsal; left (L) and right (R) sides are indicated in panel A. In both ntl and tbx16 mutant embryos, perinodal spaw expression was greatly reduced or absent (arrow in panels B, C) and LPM expression was left-sided, right-sided (arrowhead in panels B and C) or bilateral (see Table 1). (D–F) At 17–19 SS spaw remained left-sided in wild-type embryos (D) and randomized in tbx16 mutants (E), but was bilateral in all ntl mutants (F) (see Table 1). (G–I) The nodal antagonist charon is expressed around KV in wild-type embryos (arrow in panel G) and tbx16 mutants (arrow in panel H), but is reduced or absent in ntl mutant embryos (arrow in panel I). EXPRESSION / LABELING:
PHENOTYPE:
|
|
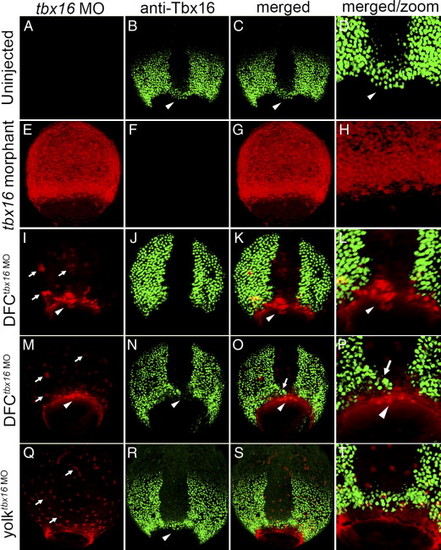
Tbx16 expression in tbx16 morphant, DFCtbx16 MO and yolktbx16 MO embryos. All panels are dorsal views between 70% and 90% epiboly stages. In uninjected embryos (A–D), fluorescent immunostaining detected Tbx16 protein (green) in margin cells, paraxial mesoderm and DFCs (arrowhead in panels B–D). In Tbx16 morphants (E–H), tbx16 MO was distributed to all embryonic cells (E) and Tbx16 expression was greatly reduced or absent (F). Two representative DFCtbx16 MO embryos are shown (I–P), in which tbx16 MO localized to nuclei in the yolk cell (arrows in panels I, M) and loaded into DFCs (arrowheads in panels I, M). In a portion of DFCtbx16 MO embryos (I–L), tbx16 MO blocked Tbx16 expression in all DFCs without affecting its expression in margin cells or paraxial mesoderm. In other DFCtbx16 MO embryos (M–P), Tbx16 expression was abrogated in a subset of DFCs (arrowhead in panels O, P), but remained normal in other DFCs (arrow in panels O, P). In yolktbx16 MO embryos, tbx16 MO localized to yolk nuclei (arrows in panel Q), but did not enter DFCs or affect Tbx16 expression in DFCs (arrowhead in panel N) or other embryonic cells. Panels D, H, L, P and T show a close-up of DFCs from merged images. EXPRESSION / LABELING:
|
|
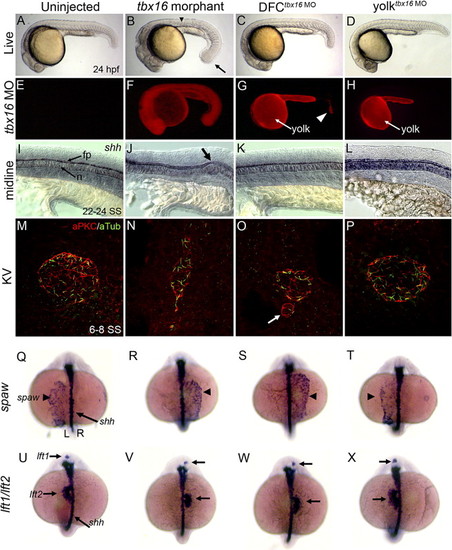
Tbx16 functions cell-autonomously in DFCs to control KV morphogenesis and LR development. (A–D) Live embryos at 24 hpf. (B) tbx16 morphants show defects seen in tbx16 mutants including a ‘spade’ tail (arrow in panel B) and trunk defects (arrowhead in panel B). In contrast, DFCtbx16 MO (C) and yolktbx16 MO (D) embryos appeared similar to uninjected controls (A). (E–H) Fluorescent images corresponding to (A–D). Fluorescent tbx16 MO was found in all cells in tbx16 morphants (F), but remained only in the yolk cell (arrow in panel G) and a small group of cells in the tail of DFCtbx16 MO embryos (arrowhead in panel G). tbx16 MO was restricted to the yolk cell (arrow in panel H) in yolktbx16 MO embryos (H). (I–L) sonic hedgehog (shh) marks midline structures such as the floor plate (fp) and notochord (n). Kinks in the midline often seen in tbx16 morphants (arrow in panel J) were not observed in DFCtbx16 MO (K), yolktbx16 MO (L) or uninjected (I) embryos. (M–P) aPKC/aTubulin co-immunostaining of KV cells between 6 and 8 SS. KV morphology defects were observed in DFCtbx16 MO embryos (K) similar to defects in tbx16 morphant (J) and mutant (Figs. 2G–L) embryos. Control yolktbx16 MO embryos (L) formed a normal KV similar to uninjected embryos (I). (Q–X) Left–right asymmetry was analyzed (see Table 2) by RNA in situ hybridization of spaw in LPM at 17–18 SS (Q–T) and lft1 and lft2 in precursors of the brain and heart respectively at 22–24 SS (U–X). sonic hedgehog (shh) marks the midline. Views are dorsal and left (L) and right (R) sides are indicated in panel Q. In wild-type embryos, spaw (Q) and lft1 and lft2 (U) were asymmetrically expressed on the left. Expression of these markers was altered in tbx16 morphant (R, V) and DFCtbx16 MO embryos (S, W). LR markers were unaffected in yolktbx16 MO embryos (T, X). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Ntl and Tbx16 are not dependent on one another in DFCs. (A–C) Double fluorescent immunostaining of Tbx16 (green) and Ntl (red) in wild-type embryos showed co-expression of these proteins in DFCs (arrowheads) between 70% and 90% epiboly. Tbx16 expression was not detected in tbx16 morphants (D), but Ntl expression in DFCs was unaffected (E). In ntl morphants, Tbx16 was expressed in DFCs (G) in the absence of Ntl protein (H). EXPRESSION / LABELING:
|
|
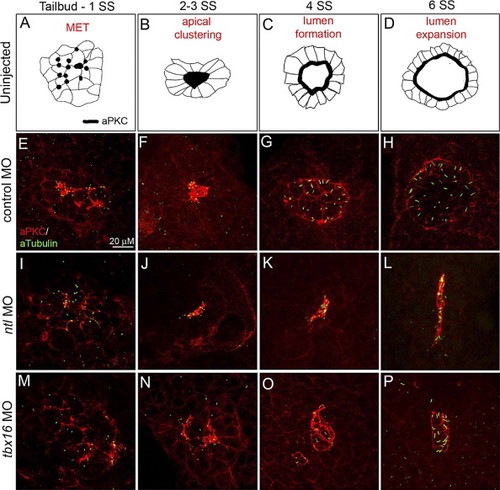
Ntl and Tbx16 regulate distinct steps of KV development. Co-immunostaining of aPKC and aTubulin in KV cells (E–P) at four time-points defined temporally distinct steps of KV development in wild-type (represented as illustrations in panels A–D) and control MO injected embryos (E–H). These steps include a mesenchymal to epithelial transition (MET) marked by apical membrane biogenesis in KV cells (A, E), aggregation of apical membranes into a central cluster (B, F) and formation (C, G) and expansion (D, H) of the fluid-filled KV lumen. For simplification, cilia were excluded from the diagrams in panels A–D. In ntl morphant embryos, apical membranes were established (I) and clustered (J), but the KV lumen failed to inflate (K, L). Apical foci were present in tbx16 morphants (M), but KV cells did not aggregate (N). Lumen formation proceeded without apical clustering, giving rise to dysmorphic and/or multiple vesicles (O, P). All confocal micrographs are at the same scale (see scale bar in panel E). PHENOTYPE:
|
|
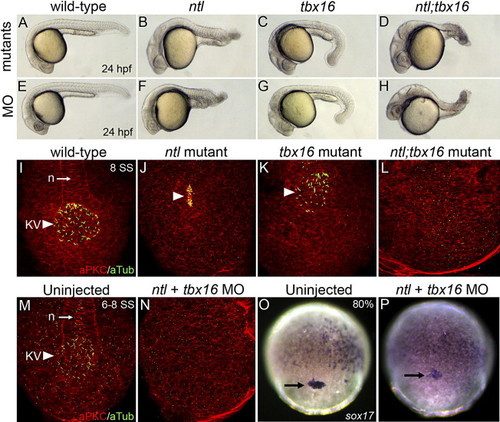
Differentiated KV cells are absent in ntl;tbx16 double mutant and morphant embryos. (A–H) At 24 hpf, ntl mutants (B), tbx16 mutants (C) and ntl;tbx16 double mutants (D) resembled embryos injected with ntl MO (F), tbx16 MO (G) and ntl + tbx16 MO (H), respectively. (I–N) aPKC/aTubulin immunostaining of KV cells at 6–8 SS. Differentiated KV cells (arrowheads in panels I–K, M) were present in wild-type (I, M), ntl mutant (J) and tbx16 mutant (K) embryos and showed characteristic morphologies (see Fig. 2). In contrast, aPKC/aTubulin positive cells were not detected in ntl;tbx16 double mutants (L) or ntl + tbx16 MO embryos (N). (O–P) sox17 RNA expression was present in DFCs (arrow in panels O and P) in uninjected controls (O) and ntl + tbx16 morphants (P) at 70–80% epiboly stages. n = notochord. EXPRESSION / LABELING:
PHENOTYPE:
|
|
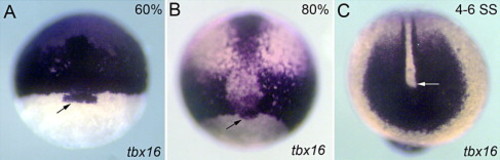
tbx16 RNA is expressed in DFCs during gastrulation. At 60% epiboly (A) and 80% epiboly (B) RNA in situ hybridization detected tbx16 expression in prechordal plate, paraxial mesoderm, margin cells and DFCs (black arrows). At 4–6 SS (C) tbx16 was strongly expressed in the tailbud (white arrow), making it difficult to determine whether tbx16 was also expressed in KV cells. EXPRESSION / LABELING:
|
|
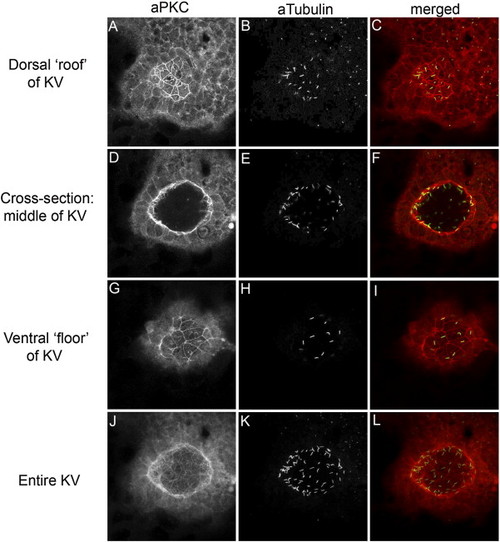
aPKC is a marker of KV cells. (A–L) Confocal microscopy of double fluorescent immunostaining in a wild-type embryo using anti-aPKC (red) and anti-acetylated Tubulin (green) antibodies shows that aPKC localizes to the apical membrane of ciliated KV cells. Confocal cross-sections through the dorsal ‘roof’ (A–C), mid-section (D–F) and ventral ‘floor’ (G–I) of KV demonstrate this apical staining on the surfaces lining the KV lumen. Additionally, each of these surfaces is ciliated. (J–L) The sum of all confocal sections through KV. |
|
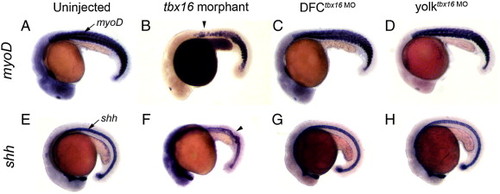
DFCtbx16 MO embryos develop normal trunk and midline structures. tbx16 morphant embryos (B, F) developed global loss-of-function tbx16 phenotypes, including trunk defects revealed by RNA staining of somites with myoD (arrowhead in B) and kinks in the midline (arrowhead in F) marked by sonic hedgehog (shh) staining. Similar to uninjected controls (A, E), normal trunk and midline development was observed in DFCtbx16 MO (C, G) and yolktbx16 MO embryos (D, H). EXPRESSION / LABELING:
|
|
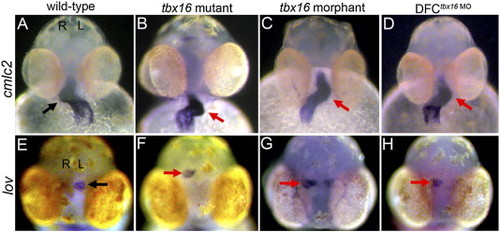
DFCtbx16 MO embryos have heart and brain laterality defects. In wild-type embryos (A, E), the heart tube, marked by cmlc2 staining, is looped to the right (arrow in A) and leftover (lov) is expressed on the left side of the brain (arrow in E). All panels are frontal views; left (L) and right (R) sides are indicated in (A, E). Heart and brain laterality was often reversed in DFCtbx16 MO embryos (D, H), similar to tbx16 mutant (B, F) and morphant (C, G) embryos. |

Unillustrated author statements EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 310(2), Amack, J.D., Wang, X., and Yost, H.J., Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer's vesicle in zebrafish, 196-210, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.