- Title
-
Obscurin is required for the lateral alignment of striated myofibrils in zebrafish
- Authors
- Raeker, M.O., Su, F., Geisler, S.B., Borisov, A.B., Kontrogianni-Konstantopoulos, A., Lyons, S.E., and Russell, M.W.
- Source
- Full text @ Dev. Dyn.
|
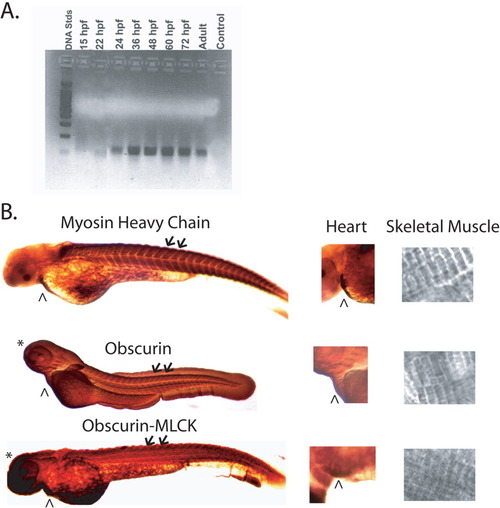
Expression of obscurin and obscurin-MLCK in developing zebrafish embryos. A: RT-PCR of total RNA from zebrafish embryos using primers from the 5' end of the obscurin/obscurin-MLCK gene. RNA samples were prepared from whole embryos 15, 22, 24, 36, 48, 60, and 72 hr post-fertilization (hpf) and from adult zebrafish hearts. Expression is first noted in embryos approximately 22 hpf with expression persisting in the adult heart. B: Immunolocalization of obscurin/obscurin-MLCK expression using the link7 and Ank antibodies in zebrafish embryos at 48 hpf. Note the expression of obscurin and obscurin-MLCK in the heart (∧) and skeletal muscle (arrows and inset, far right). Immunolocalization of myosin (MF20 antibody), which decorates the A bands of striated muscle, is shown for comparison. Note that, unlike myosin, both obscurin and obscurin-MLCK are also expressed in non-muscle tissues, including the central nervous system (*), at this stage in development. EXPRESSION / LABELING:
|
|
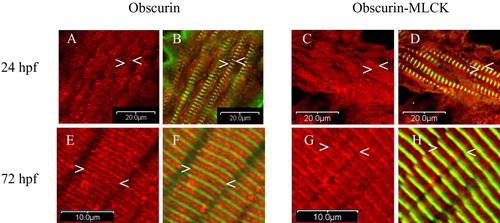
Cellular distribution of obscurin and obscurin-MLCK in zebrafish skeletal muscle during development. Embryos were fixed at 24 (A-D), and 72 (E-H) hpf and co-immunolabeled with antibodies to the ankyrin binding domain of obscurin (Ank) (red: A,B,E,F) or the carboxy terminal kinase domain of obscurin-MLCK (link7) (red: C,D,G,H) and α-actinin (green: B,D,F,H). At 24 hpf, while most of the cellular obscurin and obscurin-MLCK remains diffusely localized, some begins to organize around the Z (A-D:<) and M bands (A-D:>) of the maturing myofibrils. It is important to note that all myofibrils with a striated pattern of α-actinin staining also demonstrate organization of some of the obscurin and obscurin-MLCK around the M and Z bands. Later in development, by 72 hpf, both obscurin and obscurin-MLCK demonstrate a more distinct striated pattern with obscurin more concentrated at the M bands (E-H:>) and obscurin-MLCK at the Z bands (E-H:<). Similar results were obtained using antibodies to the amino terminal immunoglobulin domains of obscurin (4A8) and the internal kinase domain of obscurin-MLCK (SKII). Scale bars = 20 (A-D) and 10 (E-H) μm. EXPRESSION / LABELING:
|
|
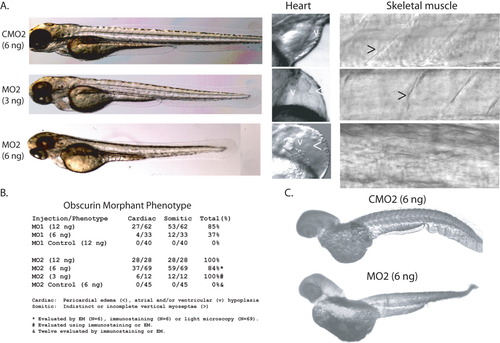
Suppression of obscurin expression in MO2-treated embryos. A: Embryos injected with MO2 (MO2; 3 and 6 ng) or a control morpholino (CMO2; 6 ng) were examined at 72 hpf. Note that the morphant embryos are slightly shorter with diminished regularity and definition of the transverse myoseptae (>) and moderate pericardial edema (<). Most 72-hpf MO2-treated embryos demonstrated a looped but hypoplastic heart with a small, underdeveloped ventricular (v) chamber compared to control. B: Phenotypic effects of injection with MO1, MO2, or the corresponding control morpholinos, CMO1 and CMO2. Embryos were phenotyped at 72 hpf and categorized as having cardiac and/or somite defects. All embryos injected with control morpholinos at these dosages were morphologically normal. C: Suppression of obscurin expression in MO2-injected embryos (6-ng dose). MO2-injected 72-hpf embryos demonstrate reduced immunostaining for obscurin (Rho Ab) compared to controls using identical reaction conditions. PHENOTYPE:
|
|
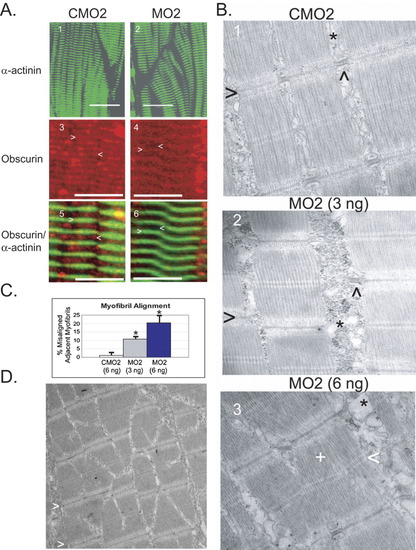
Obscurin depletion is associated with abnormalities of thick filament assembly and myofibril alignment in zebrafish embryos. At 72 hpf, embryos that had been injected with control (CMO2) or obscurin morpholino (MO2) were evaluated by immunohistochemical (A) and electron microscopic analysis (B). A: Control [CMO2 (6 ng); A.1,3,5] and obscurin morphant [MO2 (3 ng); A.2, (6 ng) A.4,6] embryos were hybridized with antibodies to α-actinin (A.1-2,5-6), and/or obscurin (Ank; A.3-6). A.5 and A.6 correspond to A.3 and A.4. Note that there irregularities of myofibril alignment and orientation in the skeletal muscle of the morphant embryos. The morphant embryos demonstrate a normal Z band structure and spacing as demonstrated by α-actinin localization, but there is decreased relative accumulation of obscurin at the M band (<) as opposed to the Z band (>) (compare A.4 and A.6 to A.3 and A.5). Scale bars = 20 μm. B: Electron micrographs of skeletal muscle from control [CMO2 (6 ng); B.1] and obscurin morphant [3 ng (B.2) and 6 ng (B.3) of MO2] embryos. Note the irregular spacing and misalignment of adjacent myofibrils in the morphant embryos. The Z bands (>) of adjacent myofibrils do not align and the sarcoplasmic reticulum (*) appears disorganized, often lacking the well-ordered triads (∧) evident in the control embryos. At the higher morpholino dose, there was occasional disorganization of the thick filaments (+) with irregularity of the M bands (<). C: Myofibril misalignment in control and morphant embryos. The percentage of myofibrils that were not aligned in register with the adjacent myofibril within the same myocyte was significantly greater in morphant than control embryos (*t-test; P < 0.01) and increased with increasing morpholino dose. D: Areas of myofibril disarray were clustered with some areas demonstrating high rates of misalignment. Note the staircase appearance of the Z bands (>) in the skeletal muscle of this 72-hpf embryo injected with 6 ng of MO2. PHENOTYPE:
|
|
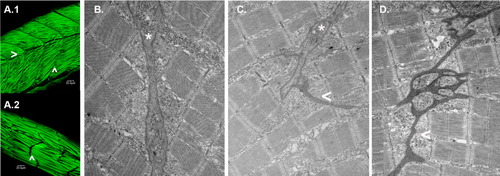
Obscurin depletion disrupts somite architecture. A: Embryos injected with MO2 (6 ng; A.2) or a control morpholino CMO2 (6 ng; A.1) were fixed and immunostained for α-actinin at 72 hpf. Note that in the morphant embryos, there are no detectable horizontal myoseptae (>) and only rudimentary transverse myoseptae (∧) compared to control embryos. Elongated, disarrayed myofibrils often extend beyond the length of a normal somite. B-D: Electron micrographs of transverse myoseptae from control [6 ng CMO2 (B)] and obscurin morphant [3 ng (C) and 6 ng (D) of MO2] embryos. Morphant embryos displayed rudimentary transverse myoseptae (C:*, C,D: <) at the ends of the skeletal myocytes compared to the well-organized transverse myoseptae of control embryos (B: *). PHENOTYPE:
|
|
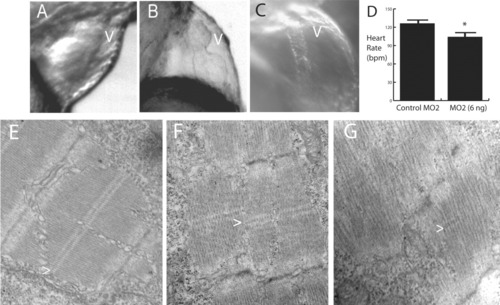
Cardiac abnormalities in morpholino-treated embryos. A-C: Morphant embryos demonstrated a spectrum of cardiac defects that ranged from mild (B) to severe (C) ventricular (v) hypoplasia. The most severely affected embryos (6 ng of MO2) studied had tube-like hearts (C). A control 72-hpf embryo is shown for comparison (A). All cardiac defects were associated with mild to marked pericardial edema. D: The heart rate of the morphant embryos (6 ng of MO2) was significantly less than that of the control embryos (*t-test; P < 0.01). As with skeletal muscle, cardiac myofibrils in morphant embryos [3 ng (F) and 6 ng (G) of MO2] were poorly aligned and organized compared to those in control-injected embryos (E). Some M bands (>) are apparent in morphant embryos but are much more irregular than in control embryos. PHENOTYPE:
|