- Title
-
In vivo Validation of Bimolecular Fluorescence Complementation (BiFC) to Investigate Aggregate Formation in Amyotrophic Lateral Sclerosis (ALS)
- Authors
- Don, E.K., Maschirow, A., Radford, R.A.W., Scherer, N.M., Vidal-Itriago, A., Hogan, A., Maurel, C., Formella, I., Stoddart, J.J., Hall, T.E., Lee, A., Shi, B., Cole, N.J., Laird, A.S., Badrock, A.P., Chung, R.S., Morsch, M.
- Source
- Full text @ Mol. Neurobiol.
|
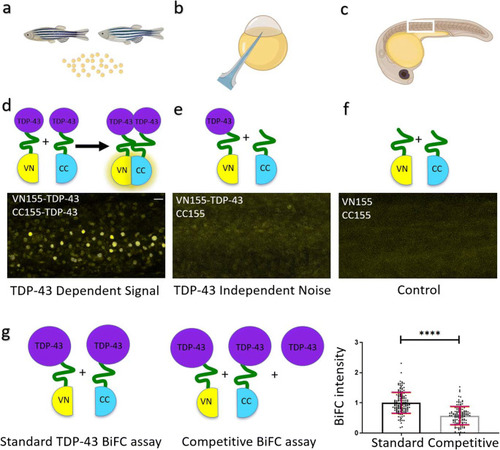
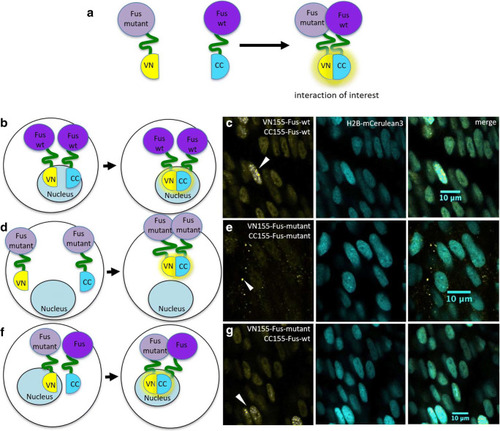
TDP-43 aggregation in zebrafish is specific. BiFC assay to determine TDP-43 aggregation. |
|
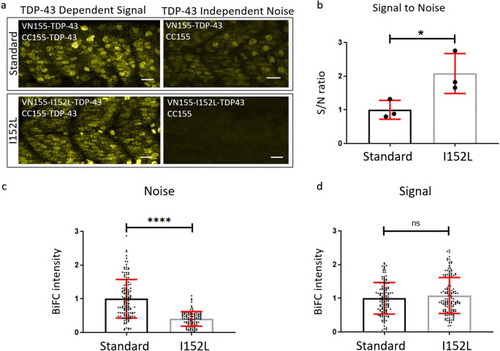
Optimized mVenus fragment (VN155-I152L) increases the signal-to-noise ratio of TDP-43 complementation. |
|
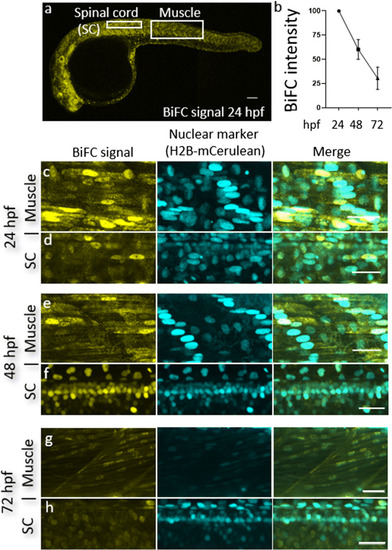
TDP-43 BiFC can be detected in muscle cells and motor neurons and is predominantly nuclear. |
|
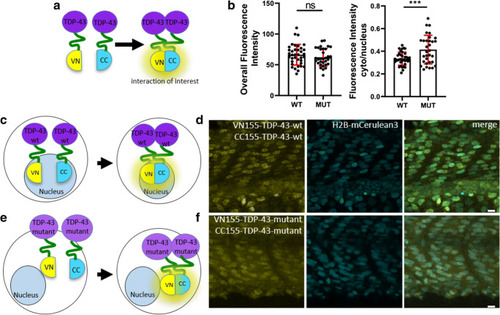
Mutant TDP-43 BiFC is mislocalized to the cytoplasm. |
|
Wild-type and cytoplasmic localized mutant Fus BiFC assay in zebrafish. |