- Title
-
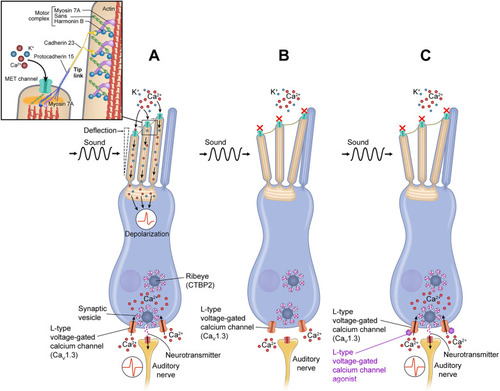
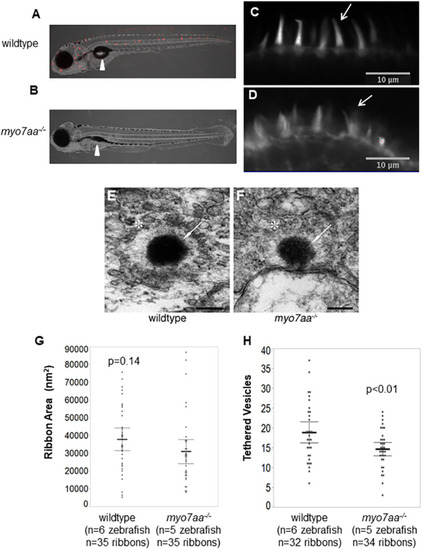
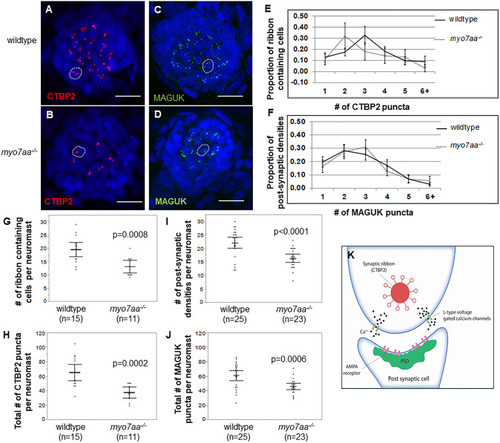
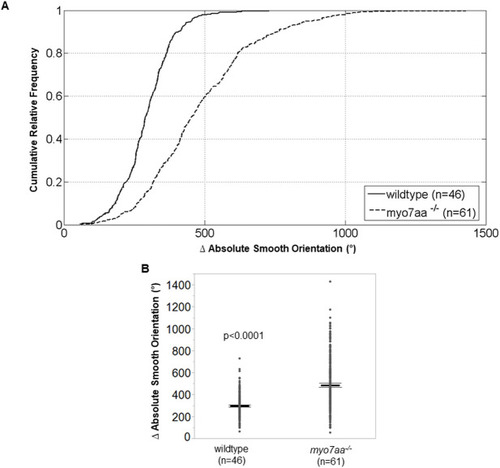
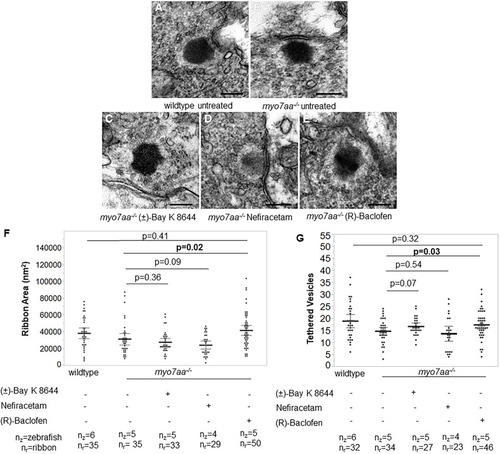
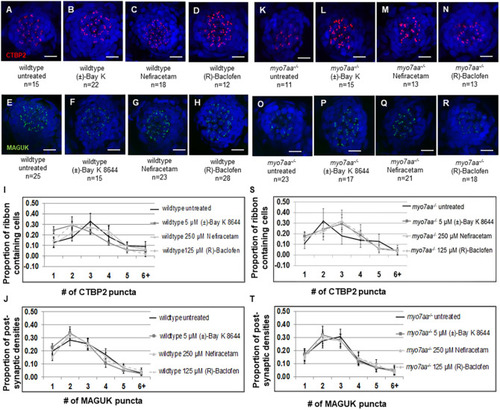
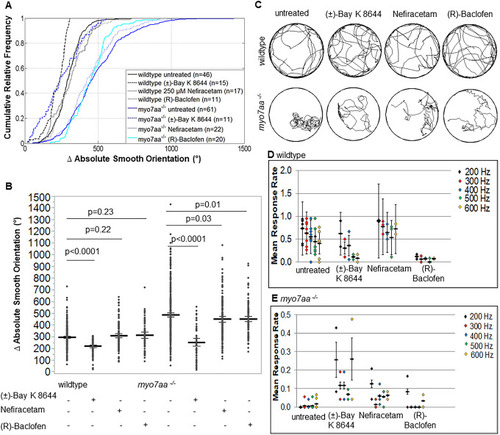
L-type voltage-gated calcium channel agonists mitigate hearing loss and modify ribbon synapse morphology in the zebrafish model of Usher syndrome type 1
- Authors
- Koleilat, A., Dugdale, J.A., Christenson, T.A., Bellah, J.L., Lambert, A.M., Masino, M.A., Ekker, S.C., Schimmenti, L.A.
- Source
- Full text @ Dis. Model. Mech.
|
|
|
|
|
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|