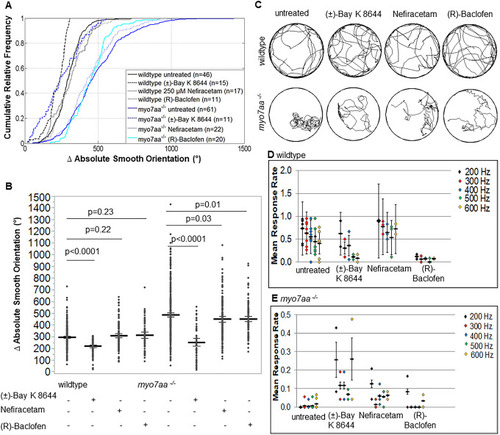
myo7aa−/− swimming behavior and acoustic startle response improvedduring treatment withL-type voltage-gated calcium channel agonists. Movement tracking of 5 dpf wild-type and myo7aa−/− larvae over a 2.5-min interval with a 5 ms electric stimulus (50 mV) administered every 20 s. Ctrax software was used for video processing and MATLAB 2012b for video analysis. (A,B) 250 µM Nefiracetam and 125 µM (R)-Baclofen did not affect the absolute smooth orientation (global change in body orientation) of wild-type larvae; however, 5 µM (±)-Bay K 8644 resulted in a decreased absolute smooth orientation compared to that of untreated wild-type larvae. When myo7aa−/− larvae were individually treated with 5 µM (±)-Bay K 8644, 250 µM Nefiracetam or 125 µM (R)-Baclofen, either treatment decreased absolute smooth orientations, with the most robust response observed during incubation with 5 µM (±)-Bay K 8644. Individual turning angles from a population of wild-type and myo7aa−/− larvae were used to construct the lines (two-tailed t-test). Bold black lines represent the mean of the data sets and error bars are 95% confidence intervals. Experiments were conducted twice for each drug treatment. (C) Sample movement tracing of wild-type and myo7aa−/− larvae within individual treatment groups indicates that in the presence of 5 µM (±)-Bay K 8644, 250 µM Nefiracetam or 125 µM (R)-Baclofen induces changes to the swimming of myo7aa−/− larvae that include smoother trajectories with more zig-zag-like swimming and fewer circling episodes. Diameter of the wells was 20 mm. (D,E) Acoustic startle response was captured by administering three stimuli at each frequency per experiment. Videos were scored blindly and the mean number of responses per total stimuli (mean response rate) was determined. myo7aa−/− larvae showed little to no response at all frequencies. Upon incubation with 5 µM (±)-Bay K 8644 acoustic startle response in wild-type larvae decreased at all frequencies; however, myo7aa−/− larvae showed a significant increase, with a response rate of >20% at 200 and 600 Hz. Incubation in 250 µM Nefiracetam increased acoustic startle response in wild-type and myo7aa−/− larvae at all frequencies, although the increase in the myo7aa−/− larvae is modest. 125 µM (R)-Baclofen decreased acoustic startle response significantly in wild-type larvae and had little to no effect on acoustic startle in the myo7aa−/− larvae. Bold black lines represent the mean of the data set and error bars are the mean variance. Experiments were conducted eight times for wild-type and myo7aa−/− mutant controls, twice for wild-type and myo7aa−/− mutants incubated in 5 µM (±)-Bay K 8644 or 125 µM (R)-Baclofen, three times for wild type incubated in 250 µM Nefiracetam, and twice for myo7aa−/− larvae incubated in 250 µM Nefiracetam.
|

