- Title
-
Imaging epilepsy in larval zebrafish
- Authors
- Burrows, D.R.W., Samarut, É., Liu, J., Baraban, S.C., Richardson, M.P., Meyer, M.P., Rosch, R.E.
- Source
- Full text @ Eur. J. Paediatr. Neurol.
|
|
|
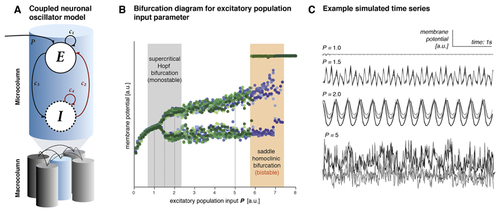
(A) Multiple Wilson-Cowan type neural mass models of excitatory and inhibitory populations were heterogeneously coupled and simulated at different values of excitatory population input |
|
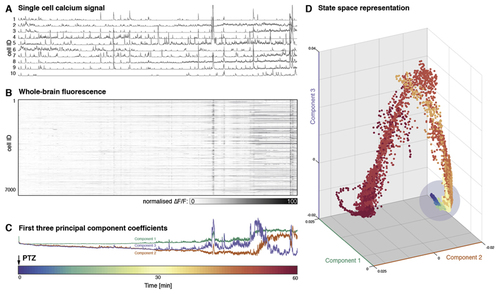
(A) Example normalised fluorescence traces are shown for individual neurons for 1 h after addition of PTZ to the bath at time point 0 showing an increase of amplitude and frequency of neuronal firing events. (B) Firing of all >7000 active cells captured in this recording. (C) First three principal components over time varying fluorescence matrix shown in (B). These indicate both a persistent drift in components 2, and 3, as well as drastic changes in the loading of all components towards the end of the recording. (D) The same data is shown as a state space plot. Whilst most of the data points exist in a restricted region/state (indicated by blue circle), the late seizure is characterised by very different activity distribution readily apparent in this low-dimensional projection as points outside of the earlier range. (Time scale for all figures shown as colour bar at the bottom of the figure). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.) |
|
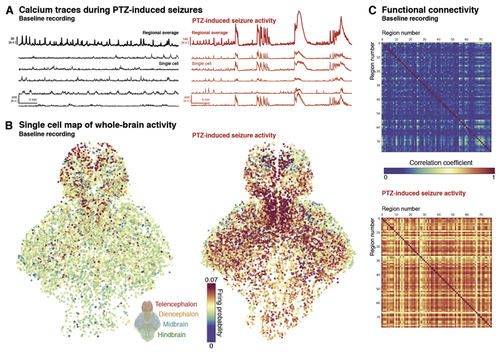
(A) Example normalised fluorescence traces are shown as whole brain mean traces and examples of individual neurons at baseline (black) and PTZ-induced seizure (red) conditions (note the difference in amplitude scale). (B) Map of individual cell firing probabilities across the two conditions demonstrates an increase in firing probability in the PTZ-induced seizure condition, with regional heterogeneity across the larval fish brain. (C) Between-region functional connectivity shown as correlation matrix. Segmented cells were registered to a zebrafish brain atlas and then averaged, to measure correlation between major brain regions (>70) across baseline and PTZ conditions. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.) |
|
(A) Coupling parameters (time constants Figure reproduced with permission from Ref. [ |