- Title
-
An interhemispheric neural circuit allowing binocular integration in the optic tectum
- Authors
- Gebhardt, C., Auer, T.O., Henriques, P.M., Rajan, G., Duroure, K., Bianco, I.H., Del Bene, F.
- Source
- Full text @ Nat. Commun.
|
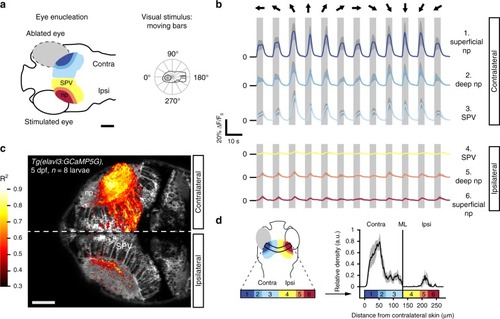
Visually evoked activity in the deep neuropil of the tectal hemisphere ipsilateral to the visually stimulated eye. |
|
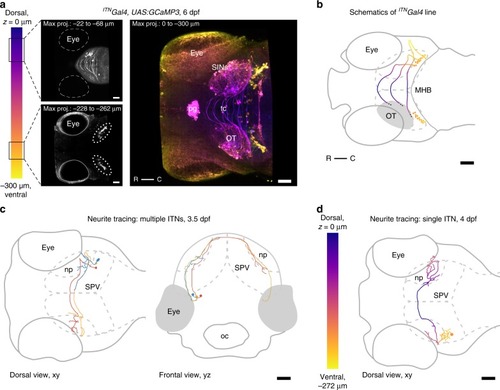
Intertectal neurons connect the two hemispheres of the optic tectum. |
|
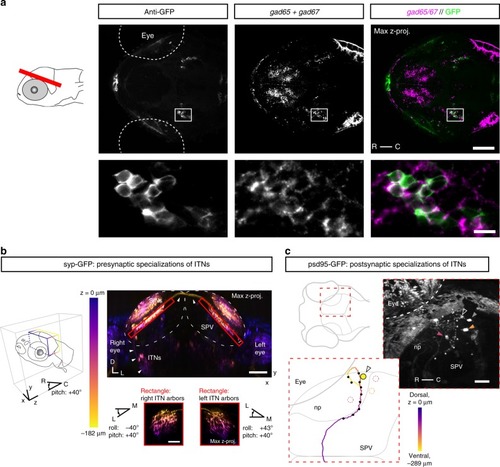
Intertectal neurons are GABAergic interneurons and form pre- and post-synaptic specializations in the OT. |
|
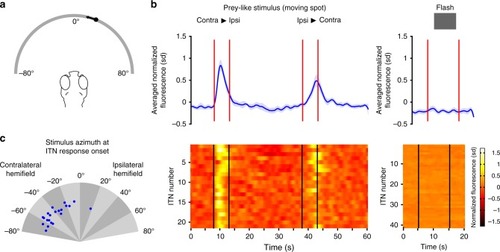
ITNs display visual sensory responses to prey-like moving target stimuli. |
|
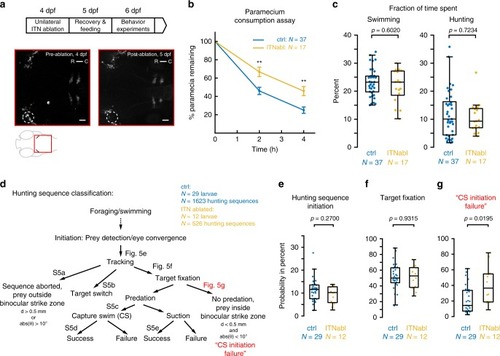
ITNs are required for the initiation of capture swims when prey is positioned in the binocular strike zone. |
|
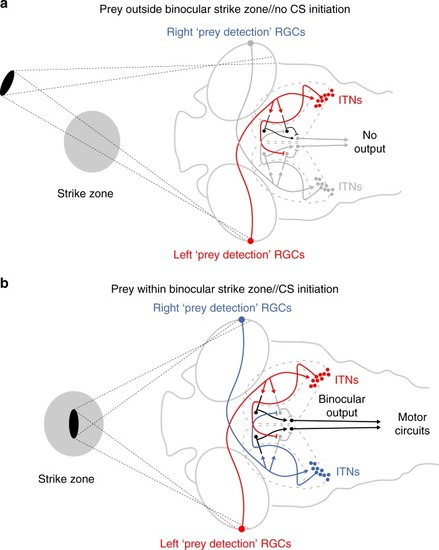
Model circuit for the integration of binocular visual input to localize prey and initiate capture swims. |