- Title
-
Interactions between the circadian clock and TGF-β signaling pathway in zebrafish
- Authors
- Sloin, H.E., Ruggiero, G., Rubinstein, A., Smadja Storz, S., Foulkes, N.S., Gothilf, Y.
- Source
- Full text @ PLoS One
|
Smad3a mRNA shows a circadian clock-controlled expression in zebrafish larvae heads. Smad3a mRNA exhibits a circadian rhythm expression pattern in zebrafish larvae heads, with mRNA expression significantly affected by sampling time (p<00.1, two-way ANOVA), showing higher expression levels during late night-time and daytime than early night-time. This pattern persists in constant darkness, suggesting that it is regulated by the circadian clock. In addition, Smad3a mRNA expression is also significantly affected by lighting conditions (p<0.001, two-way ANOVA), with a significant interaction between sampling time and light conditions (p<0.001, two-way ANOVA) (n = 15/group). (A) Top panel: schematic representation of the experimental design. The horizontal bars represent the lighting conditions before and during sampling; white boxes represent light and black boxes represent dark periods. Bottom panel: whole mount ISH images for Smad3a mRNA (dorsal views) of representative specimens raised under LD cycles until and during sampling or kept under DD during sampling. Circadian times are indicated for each sample. CT0 corresponds to “subjective lights on”, CT12 to “subjective lights-off”. White bars represent light phases and black bars represent dark phases. (B) Left: quantification of signal intensities in the head of larvae under LD and DD. Values represent the mean ±SE optical densities of the head signals. White bars represent subjective day and black bars represent subjective night. Right: Different letters represent statistically different values within each photoperiodic treatment (p<0.05, one-way ANOVA, Tukey’s test). This experiment was repeated twice resulting in a similar outcome. The represented results are of one experiment. |
|
Per1b mRNA circadian expression pattern in zebrafish larvae is phase-shifted by TGF-β inhibition. Zebrafish larvae were treated with TGF-β inhibitor LY-364947 (20 μM), and the expression pattern of Per1b was evaluated by whole mount ISH. Per1b expression was detected throughout the head region and its circadian expression pattern was altered in the presence of the TGF-β inhibitor, exhibiting a phase delay of circadian expression in comparison to a control group (DMSO). Per1b mRNA expression was significantly affected by sampling time (p<0.001, two-way ANOVA), and by an interaction between treatment and sampling time (p<0.001, two-way ANOVA) (n = 15/group). (A) Schematic representation of the experimental design. The horizontal bars represent the light conditions before and during sampling; white boxes represent light and black boxes represent dark periods. Bottom panel: whole mount ISH signals for Per1b mRNA (dorsal views of the heads) of representative specimens. Grey bars represent subjective day and black bars represent subjective night. Circadian times are indicated for each sample. CT0 corresponds to “subjective lights on”, CT12 to “subjective lights-off”. (B) Left: Quantification of signal intensities in the heads of treated and control larvae. Values represent the mean ± SE optical densities of the head signals. Right: Different letters represent statistically different values within each treatment (p<0.05, one-way ANOVA, Tukey’s test). This experiment was repeated twice, resulting in similar outcomes. The represented results are of one experiment. |
|
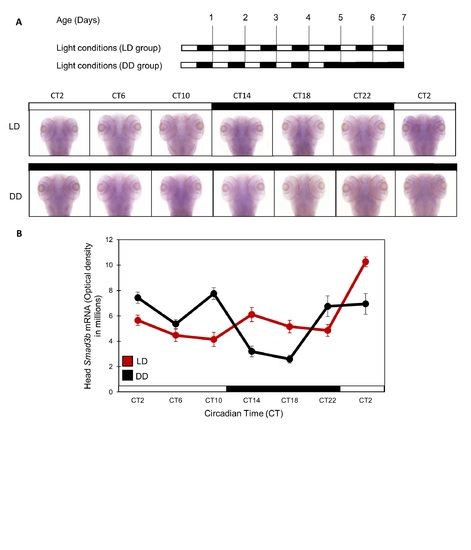
Smad3b mRNA does not show circadian expression in zebrafish larvae heads. Smad3b mRNA does not exhibit a circadian expression pattern in zebrafish larvae heads or other tissues. (A) Top panel: schematic representation of the experimental design. The horizontal bars represent the lighting conditions before and during sampling; white boxes represent light and black boxes represent dark periods. Bottom panel: Whole-mount ISH signals for Smad3b mRNA (dorsal views of the heads) of representative specimens raised under LD cycles until and during the sampling (LD group), or raised in DD during the sampling. Circadian times are indicated for each sample. CT0 corresponds to "subjective lights on", CT12 to "subjective lights-off". White bars represent light phases and black bars represent dark phases. (B) Quantification of signal intensities in the head of LD and DD larvae (n = 15/group). Values represent the mean ± SE optical densities of the head signals. White bars represent subjective day and black bars represent subjective night. |