- Title
-
ETS transcription factor Etsrp / Etv2 is required for lymphangiogenesis and directly regulates vegfr3 / flt4 expression
- Authors
- Davis, J.A., Koenig, A.L., Lubert, A., Chestnut, B., Liu, F., Desai, S.P., Winkler, T., Pociute, K., Choi, K., Sumanas, S.
- Source
- Full text @ Dev. Biol.
|
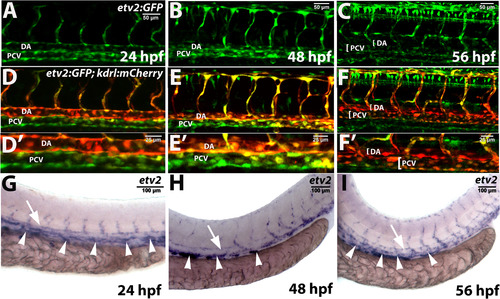
Etv2 expression in wild type embryos and larvae as analyzed by the transgenic reporter expression and in situ hybridization. (A-F) Confocal images of Tg(−2.3 kb etv2:GFP; kdrl:mCherry) reporter line at 24–56 hpf. Note that etv2:GFP expression is downregulated in the dorsal aorta (DA) at 48–56 hpf but is present in multiple cells in the posterior cardinal vein (PCV). (A-C) and (D-F) show the same embryos and larvae in green and overlaid green and red channels. (D′-F′) are higher magnification images of the DA and PCV from embryos and larvae in (D-F). Non-specific expression of etv2:GFP in neural cells is also apparent in (C,F). (G-I) In situ hybridization analysis of etv2 expression at 24–56 hpf. Note the enriched etv2 expression in the PCV (arrowheads) as compared to the DA (arrows) at 48–56 hpf. Each assay has been repeated at least twice with over 10 embryos or larvae analyzed in each group. |
|
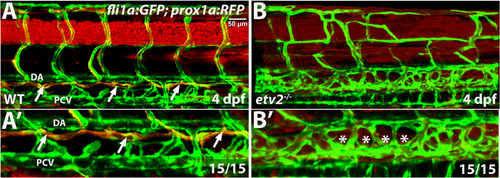
Thoracic duct is absent in etv2 mutant larvae. Confocal microscopy analysis of the thoracic duct (arrows) in wild-type siblings and etv2 mutant larvae in fli1a:GFP; prox1a:RFP background at 4 dpf. Note the absence of the thoracic duct (asterisks) and abnormal vascular patterning in etv2 mutants. (A′,B′) Higher magnification images of larvae in A,B. DA, dorsal aorta, PCV, posterior cardinal vein. |
|
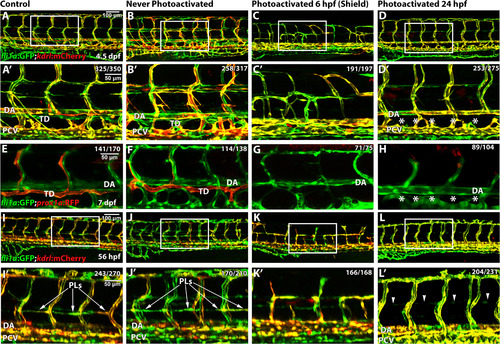
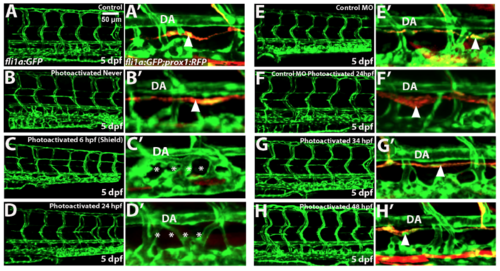
Etv2 depletion by caged MO photoactivation inhibits lymphangiogenesis. (A-H) Etv2 depletion by caged MO photoactivation inhibits thoracic duct development in fli1a:GFP; kdrl:mCherry (A-D′) and TgBAC(prox1a:KalT4-UAS:uncTagRFP) (E-H) reporter zebrafish larvae at 4.5 dpf and 7 dpf, respectively. (A,E) Control uninjected larvae with normal thoracic duct (TD) below the dorsal aorta (DA). (B,F) Embryos injected with etv2 MO and caging strand solution (hereafter called “photomorph solution”), but never exposed to UV photoactivation, develop normal thoracic duct similar to uninjected controls. (C,G) Embryos injected with photomorph solution and exposed to UV photoactivation at 6 hpf (shield stage), develop abnormal vasculature (C) and no or discontinuous thoracic duct (G) compared to control embryos as expected. (D,H) Embryos injected with photomorph solution, exposed to UV photoactivation at 24 hpf, develop normal vasculature compared to control embryos but discontinuous or absent TD (asterisks). (I-L′) Etv2 is required for the formation of parachordal lymphangioblasts, as observed in fli1a:GFP; kdrl:mCherry reporter zebrafish larvae at 56 hpf. (I) Control uninjected larvae. (J) Embryos injected with photomorph solution, but never exposed to UV photoactivation, develop normal parachordal lymphangioblasts (PL, arrowheads) similar to uninjected controls. (K) Embryos injected with photomorph solution, and UV photoactivated at 6 hpf (shield stage), develop abnormal vasculature, while PLs cannot be visualized due to abnormal branching of ISVs. (L) Embryos injected with photomorph solution, and UV photoactivated at 24 hpf, develop normal vasculature compared to control embryos while PLs are absent (arrowheads). A′-D′ and I′-L′ show magnified areas indicated by boxes in A-D and I-L. Each experiment has been repeated at least three times. Number of larvae showing a displayed phenotype out of the total number of larvae is shown in the upper right corner. |
|
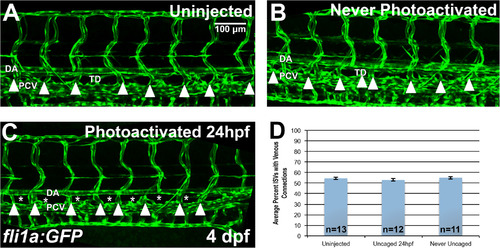
Etv2 depletion by caged MO photoactivation inhibits lymphangiogenesis but not the formation of venous intersegmental vessels (ISVs). (A-D) Analysis of venous ISVs in fli1a:GFP larvae at 4 dpf. (A) Control uninjected larva at 4 dpf, with the thoracic duct (TD) located below the dorsal aorta (DA). Embryos injected with photomorph solution and never photoactivated develop normal TD and vasculature (B), while injected embryos UV photoactivated at 24 hpf (C), develop normal vasculature compared to control embryos, while the thoracic duct is absent (asterisks) at 4 dpf. Note that the venous derived intersegmental vessels are present in both control and Etv2-inhibited larvae (arrowheads). (D) Quantification of percent of ISVs with venous connection at 4 dpf in control uninjected larvae (54.5 ± 1.2%, n = 13) compared to never photoactivated (55.1 ± 1.2%, n = 11, p = 0.74, two tailed Student's t-test) and 24 hpf photoactivated (52.9 ± 1.2%, n = 12, p = 0.34, two tailed Student's t-test) larvae, indicates no significant difference between groups. Mean±SEM is shown. Individual data points are shown in Fig. S4A. This experiment has been replicated 3 times. |
|
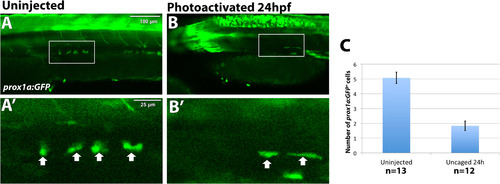
Etv2 depletion by caged MO photoactivation results in reduction of prox1a:GFP+ lymphatic progenitor cells. TgBAC(prox1a:KalT4-UAS:uncTagRFP); Tg(UAS:GFP) live embryos were imaged by confocal microscopy at 32 hpf. The number of prox1a:GFP+ cells is significantly reduced, 1.8 ± 0.32 cells, in embryos where etv2 is depleted at 24 hpf by caged MO photoactivation (n = 12) (B), compared to uninjected embryos (A) (5.1 ± 0.38 cells, n = 13, p < 0.0001, two tailed student's t-test). Arrows indicate prox1a:GFP+ endothelial cells. (C) Quantification of prox1a:GFP positive cells across the width of 9 somites in the trunk region. Mean±SEM is shown. Individual data points are shown in Fig. S4B. This experiment has been replicated twice. |
|
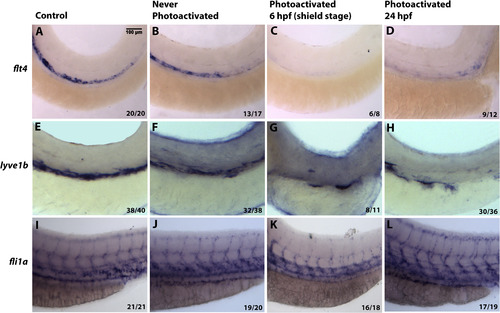
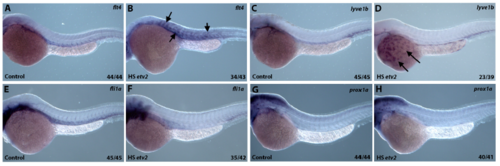
Inducible Etv2 knockdown results in decreased expression of genes associated with lymphangiogenesis as analyzed by in situ hybridization at 56 hpf. (A, E, I) Uninjected larvae illustrate normal flt4, lyve1b and fli1a expression in the PCV. (B, F, J) Larvae injected with photomorph solution and kept in dark show expression pattern similar to uninjected controls. (C, G, K) Larvae injected with photomorph solution and photoactivated at the shield stage (6 hpf) show absent flt4 and lyve1b expression from the major axial vessels. fli1a expression is present in the axial vessels due to partial recovery of Etv2 knockdown phenotype observed at the later stages; however, ISV sprouting is abnormal. (D, H, L) Injected larvae photoactivated at 24 hpf, show decreased flt4 and lyve1b expression (D,H), compared to control larvae. However, note the unaffected pattern of fli1a (L), demonstrating that Etv2 knockdown at 24 hpf specifically affects markers associated with lymphangiogenesis. Analysis for each marker has been repeated at least twice. Number of larvae showing a displayed phenotype out of the total number of larvae is shown in the lower right corner. |
|
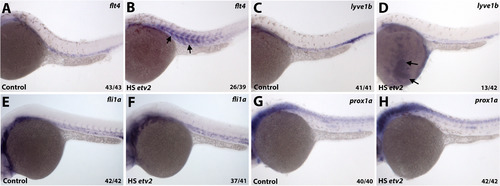
Heatshock inducible overexpression of Etv2 induces ectopic flt4 and lyve1b expression. (A,B) Embryos injected with hsp70:Etv2-mCherry overexpression construct show ectopic induction of flt4 (B) in the somites (arrows) following the heat-shock compared to uninjected controls (A). (C,D) Ectopic expression of lyve1b over the yolk is apparent in a subset of embryos that overexpress hsp70:Etv2-mCherry (arrows, D). (E-H) No ectopic induction of fli1a (E,F) or prox1a (G,H) was apparent in the embryos that overexpress hsp70:Etv2-mCherry. All embryos are at 28–30 hpf. |
|
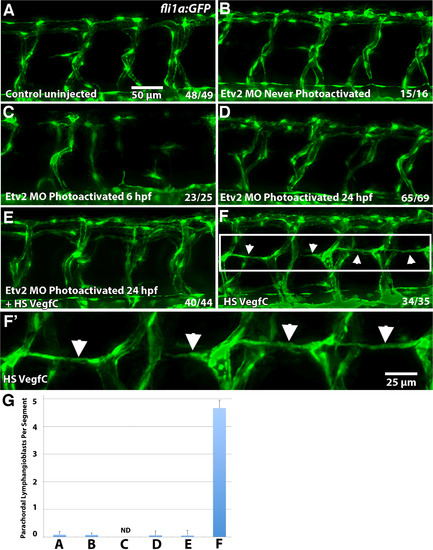
Etv2 function is required for induction of lymphangiogenesis by Vegfc. (A-F) Parachordal lymphangioblasts (PL) were analyzed at 52 hpf in fli1a:GFP transgenic line, and visualized by GFP fluorescence, while Etv2 depletion was achieved by UV photoactivation of previously described photomorph solution. (A) Control uninjected larva; (B) Etv2 photomorph injected and never photoactivated larva; (C) Etv2 photomorph injected larva, uncaged at 6 hpf (shield stage); (D) Etv2 photomorph injected larva, uncaged at 24 hpf; (E) Heat-shock Vegfc overexpressing larva, Etv2 depleted by photoactivation at 24 hpf; (F) Larvae injected with heat shock overexpression Vegfc construct only. Note that at this stage there are very few lymphangioblasts in the control larvae (A), while greatly increased number and precocious formation of parachordal lymphangioblasts is observed in Vegfc overexpressing larvae (F, arrows), which are reduced upon Etv2 depletion (C-E). (G) Average number of lymphatic precursor cells per segment. Analysis was accomplished by counting the number of parachordal lymphangioblasts in ten intersegmental spaces over the trunk of each larva per group. The average number of PLs per segment was then determined. Mean values ± SEM are shown. Note that the larvae were analyzed at 52 hpf, prior to the emergence of PLs in the control group. Larvae that were uncaged at 6 hpf (shield stage) had abnormal vasculature that made calculation of PLs impossible (ND, not determined) (C). The experiment has been replicated twice. EXPRESSION / LABELING:
|
|
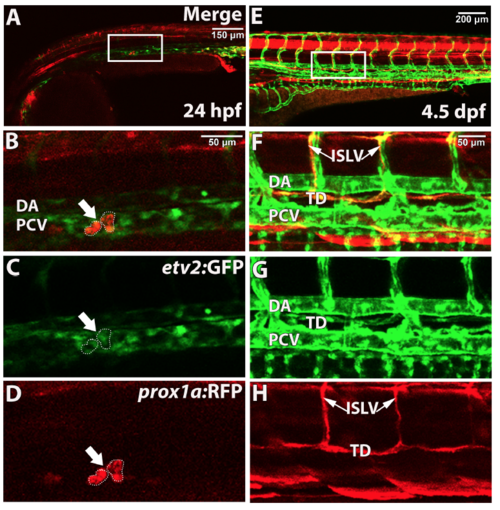
etv2 and prox1a are co-expressed in lymphatic progenitors in the posterior cardinal vein, parachordal lymphangioblasts, and the thoracic duct. Confocal imaging analysis of live embryos and larvae. TgBAC(etv2:GFP) and prox1a:RFP are co-expressed in lymphatic progenitors (arrow/dashed lines) in the PCV at 24 hpf (A-D, projection of 3 axial positions) and the thoracic duct and intersegmental lymphatic vessels at 4.5 dpf (E-H). (B-D and F-H) show magnified regions indicated by boxes in (A,E). Embryos and larvae have been analyzed from 2 independent experiments. DA, dorsal aorta; PCV, posterior cardinal vein; TD, thoracic duct; ISLV, intersegmental lymphatic vessel. |
|
Caged etv2 MO photoactivation at 24 hpf but not at the later stages specifically inhibits thoracic duct formation as analyzed by confocal imaging of fli1a:GFP; TgBAC(prox1a:KalT4-UAS:uncTagRFP) larvae at 5 dpf. (A-H) Images of the trunk region in the GFP channel (left panels) and enlarged views of the thoracic duct in GFP and RFP channels (A'-H'). DA, dorsal aorta. (A-D) Caged etv2 MO photoactivation at 24 hpf results in the absence of the thoracic duct. Note the normal vascular patterning and present thoracic duct (arrowhead) in uninjected control and never photoactivated larvae (A,A’,B,B’), abnormal vascular patterning and absent thoracic duct in the early uncaged larvae (shield stage, 6 hpf) (C, C’, asterisks) and normal vascular patterning and absent thoracic duct when Etv2 function was inhibited at 24 hpf (D, D’, asterisks). (E,F) Injection and photoactivation of the standard control MO and etv2 caging strand mixture does not affect vascular patterning or thoracic duct formation (arrowhead). (E,E’) Standard control MO / etv2 caging strand mixture injected larvae which were not photoactivated; (F,F’) larvae that were photoactivated at 24 hpf. (G,H) Photoactivation of caged etv2 MO injected embryos at 34 hpf (G,G’) or 48 hpf (H,H’) does not affect thoracic duct formation (arrowheads). Experiment has been replicated at least twice. |
|
Heatshock inducible overexpression of Etv2 induces ectopic flt4 and lyve1b expression. (A,B) Embryos injected with hsp70:etv2-mCherry overexpression construct show ectopic induction of flt4 (B) in the somites (arrows) following the heat-shock compared to uninjected controls (A). (C,D) Ectopic expression of lyve1b over the yolk is apparent in a subset of embryos that overexpress hsp70:etv2-mCherry (arrows, D). (E-H) No ectopic induction of fli1a (E,F) or prox1a (G,H) was apparent in the embryos that overexpress hsp70:etv2- mCherry. All embryos are at 52-54 hpf. |
|
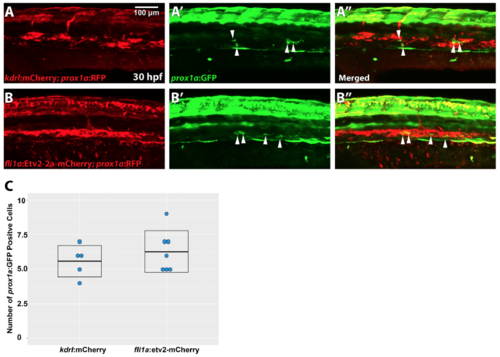
Vascular specific overexpression of Etv2 does not result in expansion of lymphatic progenitors. Injection of fli1a:etv2-2a-mCherry (B-B’’) does not result in expansion of prox1a:GFP+ cells (arrowheads) etv2 at 30hpf (5.6±0.5, n=5) compared to control injection of kdrl:mCherry (A-A’) (6.3±0.6, n=7). Quantification of number of prox1a+ cells in the vasculature (C) (two-tailed Student’s t test p= 0.41). Mean±SEM is shown. Experiment has been repeated twice. |
|
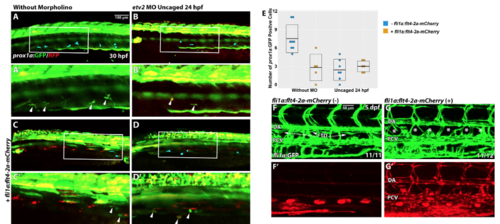
Vascular specific overexpression of Flt4 is not sufficient to rescue lymphangiogenesis defects in photoactivated etv2 knockdown embryos. (A,B) The number of lymphatic prox1a:GFP cells is reduced at 30 hpf in embryos injected with etv2 Photomorph solution and photoactivated at 24 hpf (2.4±0.6, n=7) compared to control uninjected embryos (7.5±0.8, n=8, p<0.001, two-tailed Student’s test). Note that prox1a reporter expresses both RFP and GFP. (C) Injection of fli1a:flt4-2a-mCherry results in a reduction of the number of prox1a:GFP+ cells at 30 hpf in wild type embryos (2.8±1.0, n=5, p=0.004). (D) Co-injection of fli1a:flt4-2a-mCherry together with etv2 Photomorph solution followed by photoactivation at 24 hpf results in a reduced number of prox1a:GFP cells (3±0.4, n=6, p=0.48 compared with the injection of etv2 Photomorph alone, panel B). (A’,B’,C’,D’) are higher magnification images of (A,B,C,D). (E) Quantification of prox1a:GFP cell number in the trunk region (across 9 somite length) in different groups. Each data point corresponds to the cell number in a single embryo. Mean±SEM values are shown. Arrowheads or blue pseudocolor indicate prox1a:GFP+ cells within the PCV. (F,G) Stable transgenic fli1a:flt4-2a-mCherry larvae (G,G’) have defective thoracic duct development at 5 dpf compared to fli1a:GFP larvae (F,F’). Each experiment has been repeated a minimum of two times. |
Reprinted from Developmental Biology, 440(1), Davis, J.A., Koenig, A.L., Lubert, A., Chestnut, B., Liu, F., Desai, S.P., Winkler, T., Pociute, K., Choi, K., Sumanas, S., ETS transcription factor Etsrp / Etv2 is required for lymphangiogenesis and directly regulates vegfr3 / flt4 expression, 40-52, Copyright (2018) with permission from Elsevier. Full text @ Dev. Biol.