- Title
-
State-Dependent Modulation of Locomotion by GABAergic Spinal Sensory Neurons
- Authors
- Fidelin, K., Djenoune, L., Stokes, C., Prendergast, A., Gomez, J., Baradel, A., Del Bene, F., Wyart, C.
- Source
- Full text @ Curr. Biol.
|
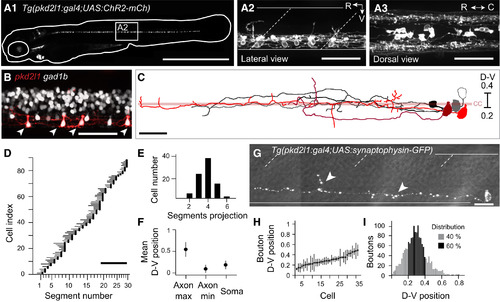
The Tg(pkd2l1:gal4)icm10 Stable Transgenic Line Specifically Labels CSF-cNs in the Zebrafish Spinal Cord (A1) Complete pattern of expression of mCherry in Tg(pkd2l1:gal4;UAS:ChR2-mCh) double transgenic larvae at 4 dpf. (A2) Lateral view of the spinal cord shows that mCherry was restricted to CSF-cNs in the ventral part of the spinal cord. Seven CSF-cNs were labeled per axial segment on average. (A3) Dorsal view of the spinal cord shows that axonal projections of CSF-cNs were ipsilateral and located in the lateral margins of the spinal cord. See also Figure S1 and Table S1. (B) Overlap of mCherry (red) and GFP (gray) in the Tg(pkd2l1:gal4;UAS:ChR2-mCh;Gad1b:GFP) triple transgenic larvae confirms the GABAergic nature of CSF-cNs (arrowheads). Note that the Tg(pkd2l1:gal4) line does not label all CSF-cNs. (C) Morphology of five CSF-cNs located in segment 9 at 3 dpf after single-cell imaging and reconstruction. Axonal projections varied in length, in branching as well as in dorsoventral (D-V) positioning. Cells were aligned according to the D-V position of their cell body. The central canal (cc) is represented by the light red bar. (D) Mapping of CSF-cNs axonal projections across the rostrocaudal (R-C) axis. All CSF-cNs had ascending projections reaching from two to six segments away from the cell body (black circles, n = 88 cells). (E) Distribution of the number of segments covered by single axons of CSF-cNs. (F) Mean D-V positions of axons (maxima and minima) and soma of CSF-cNs. (G) Single Synaptophysin-GFP CSF-cN with punctate synaptophysin clustering consistent with presynaptic boutons distributed along the entire axonal arborization (arrowheads). See also Movie S1. (H) D-V position of putative synaptic boutons for CSF-cNs sorted according to their soma position along the D-V axis (n = 36 cells). (I) Distribution of putative synaptic boutons along the D-V axis (black bars indicate that 60% of the boutons are confined in the 0.2–0.4 interval). In (A2) and (G), white solid lines delineate the ventral and dorsal limits of the spinal cord; dashed lines indicate the limits of axial segments. Scale bars are 1 mm in (A1), 50 µm in (A2), (A3), and (B), 900 µm in (D), and 20 µm in (C) and (G). R, rostral; C, caudal; V, ventral; CC, central canal. (A)–(B) were reconstructed from Z projection stacks through the entire spinal cord. Data are represented here as mean ± SD. EXPRESSION / LABELING:
|
|
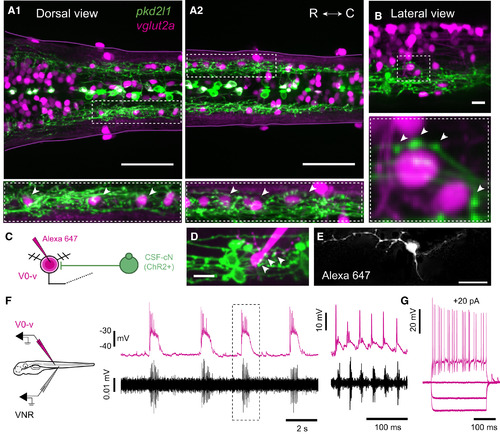
CSF-cNs Project onto V0-v Glutamatergic Interneurons (A1 and A2) Z projection stack of a few optical sections imaged from the dorsal side in Tg(pkd2l1:gal4;UAS:ChR2-YFP; vglut2a:lox-DsRed-lox-GFP) triple transgenic larvae reveal the localization of glutamatergic neurons (DsRed+, magenta) relative to CSF-cN projections (YFP+, green) in rostral (A1) and caudal (A2) spinal cord. Zoom of lateral regions circled in dashed lines show that CSF-cN projections surround the DsRed+ nucleus of ventrolateral glutamatergic interneurons (arrowheads in bottom panels, A1 and A2). See also Table S1. (B) Apposition of CSF-cN axonal varicosities onto the cell bodies of two ventrolateral vglut2a+ interneurons (arrowheads). (C) Ventrolateral vglut2a+ V0-v interneuron receiving projections from CSF-cNs were filled with Alexa 647 in order to image and reconstruct their morphology. (D) Z projection stack of a few optical sections imaged from the lateral side in a Tg(pkd2l1:gal4;UAS:ChR2-YFP;vglut2a:lox-DsRed-lox-GFP) triple transgenic larva labeling CSF-cNs (ChR2-YFP+, green) and glutamatergic interneurons (DsRed+, magenta) after dye filling a vglut2a+ V0-v interneuron. Arrowheads highlight axonal projections of CSF-cNs onto the soma and dendrites of the filled V0-v interneuron. (E) Typical morphology of ventrolateral vglut2a+ V0-v interneurons filled with Alexa 647. (F) Paired ventral nerve root recording (VNR) with whole-cell current clamp recording of a V0-v interneuron showing rhythmic activity during every episode of fictive slow locomotion (burst frequency ranged between 15 and 30 Hz). The region circled with dashed line is zoomed in on the right to emphasize the activity of V0-v during a fictive bout. V0-v action potentials preceded motor neurons spiking for all locomotor bursts. (G) V0-v cells are electronically compact, typically requiring only 10–20 pA of current injection to reach action potential (AP)-firing threshold. Note that the cell depicted in (G) is not the same cell as in (F). Scale bars are 50 µm in (A1) and (A2) and 10 µm in (B), (D), and (E). In each panel, Z projection stacks were reconstructed from a few optical sections (depth = 0.55 µm). EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
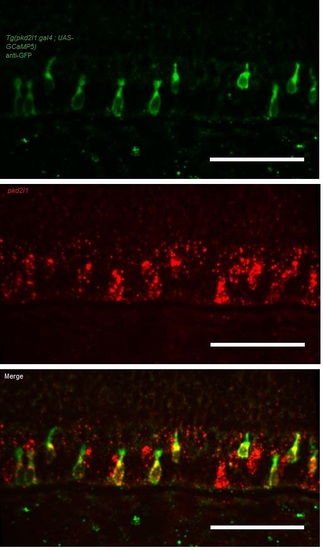
Related to Figure 1. The Tg(pkd2l1:gal4)icm10 line drives expression in pkd2l1 positive cells in the zebrafish larva. Fluorescent in situ hybridization (FISH) for pkd2l1 was combined with an immunohistochemistry against GFP in a Tg(pkd2l1:gal4;UAS :GCaMP5G) larva at 3dpf. Spinal neurons expressing GCaMP5G (green) are positive for pkd2l1 (Pkd2l1+ in red), see arrowheads, n = 5 larvae. Scale bars represent 50 µm. |