- Title
-
Mechanisms of prickle 1a function in zebrafish epilepsy and retinal neurogenesis
- Authors
- Mei, X., Wu, S., Bassuk, A.G., and Slusarski, D.C.
- Source
- Full text @ Dis. Model. Mech.
|
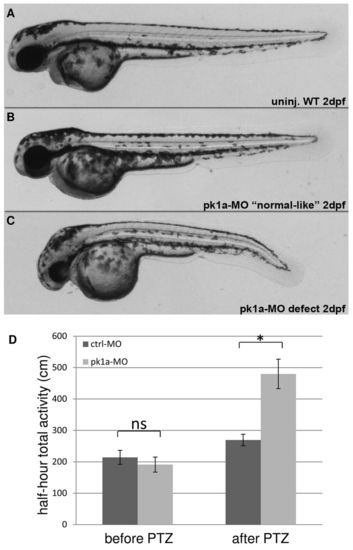
pk1a knockdown sensitizes zebrafish to PTZ treatment. (A-C) Morphology of wild-type and pk1a-MO-injected embryos at 2 dpf: wild-type uninjected embryo (A); pk1a-MO-injected embryos ranging from ‘normal-like’ (B) to curved body axis (C). (D) Graph of total half-hour activity before and after PTZ treatment for larvae injected with control-MO and pk1a-MO. Wilcoxon Rank Sum test found no significant increase (ns) in activity of control-MO and pk1a-MO injected larvae prior to PTZ treatment. There was a significant increase in activity in control after PTZ compared with control before PTZ (P<0.05) and for pk1a morphants after PTZ compared with before PTZ (P<0.05). *P<0.05 is the significant difference between control and pk1a morphant after PTZ treatment. For control-MO, n=48 and for pk1a-MO, n=48. Data presented as mean ± s.e.m. PHENOTYPE:
|
|
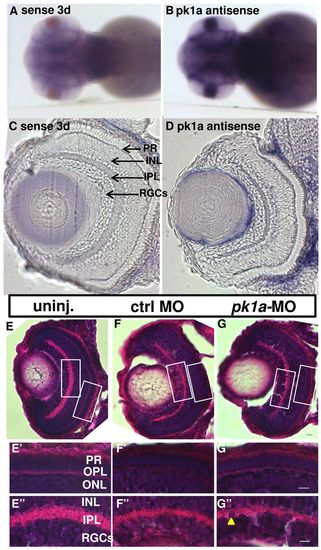
pk1a is expressed in the brain and retina and is not required for overall retinal lamination. (A,B) Whole-mount in situ hybridization of 3 dpf embryos. Images are from the dorsal view. An antisense RNA riboprobe is used to determine the expression pattern of pk1a, and a riboprobe from the sense strand is used as a control. (C,D) Sections of the eye after whole-mount in situ hybridization at 3 dpf: sense control (C) and pk1a antisense (D). Arrows show different layers in the retina. (E–G′′) H&E staining of eye sections at 76–78 hpf. (E–G) Whole retina sections of uninjected (E), control-MO-injected (F) and pk1a-MO-injected larvae (G); magnification 40×. (E′–G′′) Enlarged areas of the white boxes in E–G; the arrowhead in G′′ indicates nuclei disorganization; magnification 63×. PR, photo-receptors; INL, inner nuclear layer; IPL, inner plexiform layer; RGCs, retina ganglion cells; OPL, outer plexiform layer; ONL, outer nuclear layer. Scale bars: 5 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
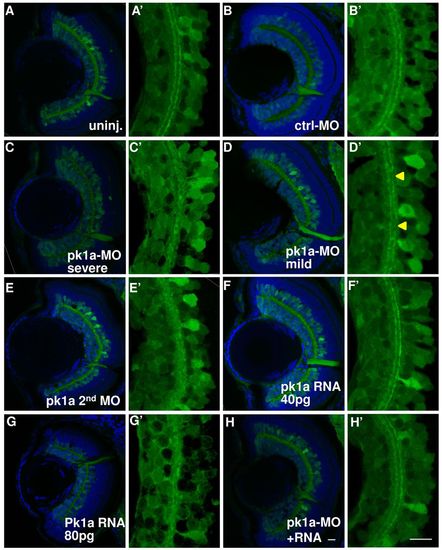
pk1a knockdown induces inner plexiform layer defects. (A–H′) Sections at 76–78 hpf in the HuC:gfp transgenic line. Green channel, GFP; blue channel, To-Pro3 nuclear counter-staining. (A–H) Whole eye visualized at 63×. Representative sections from uninjected embryos (A) and from embryos injected with control-MO (B); pk1a-MO, showing severe IPL defects (C); pk1a-MO, showing mild IPL defects (D); a second pk1a-MO (E); 40 pg pk1a RNA (F); 80 pg pk1a RNA (G); pk1a-MO plus 40 pg pk1a RNA (H). (A′–H′) Digital 3× zoom of the white boxed areas in A–H, showing the green channel only. In the uninjected (A′) and control-MO-injected embryos (B′), there are two clearly stratified tracks. pk1a morphants exhibit either loss of the organization (C′) or discontinuous tracks (arrowheads in D′). A second pk1a-MO also induced similar IPL defects (E′). Although a low dose of pk1a RNA injection does not show a defect (F′), a higher dose does (G′). The defects can be partially suppressed by a low dose pk1a RNA co-expression (H′). Scale bar: 10 μm. PHENOTYPE:
|
|
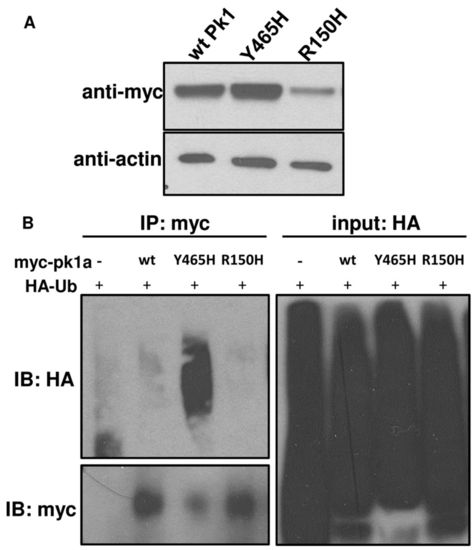
pk1a epilepsy mutants display distinct biochemical properties. (A) Western blot of myc-tagged wild-type (wtPk1) and mutant forms of Pk1a. Protein lysates were made at 80% epiboly stage. β-actin was used as a loading control. (B) Pull-down assays showing the extent to which wild-type (wt) and mutant forms are ubiquitylated. HA-Ub and pk1a RNAs were co-injected into one-cell stage embryos and at 80% epiboly total cell lysates were pulled down by anti-myc antibody beads and blotted for anti-myc or anti-HA. Embryos injected with HA-Ub without pk1a RNA were subjected to anti-myc pull-down as a negative control. |
|
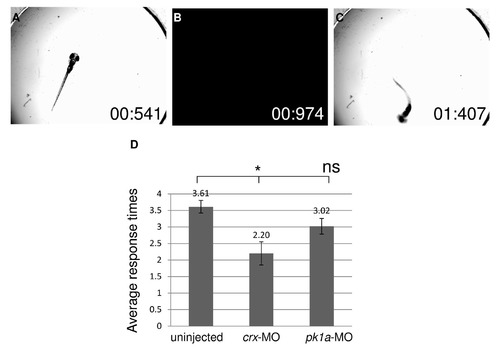
pk1a knockdown does not significantly impair visual functions. (A-C) Bright-field images selected from a movie during vision assay at 5 dpf. (A) larva under bright light illumination. (B) A short period of darkness. (C) Characteristic change of swimming direction after light change. Numbers in the images indicate the time points in “second : millisecond”. (D) Vision assay plotted by average number of times an embryo was scored as “positive” out of five trials. Wild-type uninjected embryos respond at an average of 3.6 times, while crx-MO injected embryos respond only 2.2 times. pk1a-MO morphants respond 3.0 times, which is not significantly different from wild-type embryos. *P<0.01; ns, not significant, ANOVA, uninjected n=49, crx-MO n=20, pk1a-MO n=46. PHENOTYPE:
|