- Title
-
Interaction between alk1 and blood flow in the development of arteriovenous malformations
- Authors
- Corti, P., Young, S., Chen, C.Y., Patrick, M.J., Rochon, E.R., Pekkan, K., and Roman, B.L.
- Source
- Full text @ Development
|
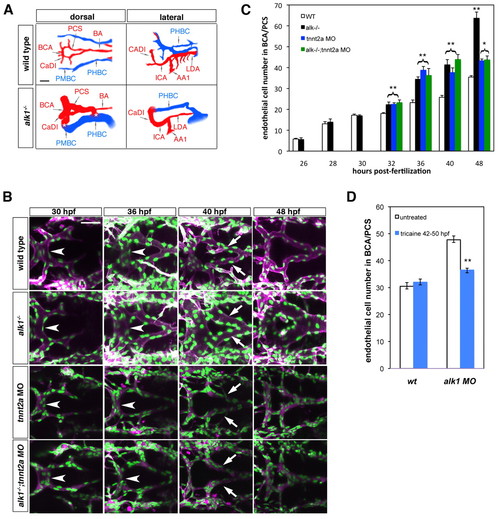
Blood flow modifies endothelial cell number in alk1 mutants. (A) Wiring diagrams of perfused head vessels in 48 hpf wild-type and alk1-/- embryos, derived from two-dimensional confocal projections of Tg(gata1:dsRed)sd2 embryos. Arteries are red; veins are blue. alk1 mutants exhibit enlarged arteries and AVMs (*) between the BCA/PMBC and/or BA/PHBC. Vessels represented in wild-type embryos but not alk1-/- embryos are present but not patent in mutants. AA1, first aortic arch; BA, basilar artery; BCA, basal communicating artery; CaDI, caudal division of internal carotid artery; ICA, internal carotid artery; LDA, lateral dorsal aorta; PCS, posterior communicating segments; PHBC, primordial hindbrain channel; PMBC, primordial midbrain channel. Scale bar: 50 μm. (B) Development of the BCA/PCSs in wild type (row 1), alk1-/- (row 2), tnnt2a morphants (MO) (row 3) and alk1-/-;tnnt2a MO (row 4). Images are two-dimensional confocal projections of live Tg(fli1a:nEGFP)y7;Tg(fli1a.ep:mRFP-F)pt505 embryos, dorsal views, anterior leftwards. Endothelial cell nuclei are green; endothelial cell membranes are magenta. In alk1-/-, the BCA (arrowhead) is enlarged by 36 hpf, and the PCSs (arrows) by 40 hpf. BCA/PCS morphology is relatively normal in tnnt2 MO and alk1-/-;tnnt2 MO, although vessels are collapsed. Scale bar: 50 μm. (C) Quantification of endothelial cell number from confocal micrographs of fixed Tg(fli1a:nEGFP)y7 embryos. alk1-/- with (black bars) or without (green bars) blood flow show similar increases in endothelial cell number compared with wild-type siblings (white bars) between 32-40 hpf, although the pronounced increase between 40 and 48 hpf observed in alk1-/- depends on blood flow. Cell number in tnnt2a MO (blue bars) is not different from cell number in alk1-/-;tnnt2a MO, suggesting that lack of flow phenocopies alk1 mutants in terms of endothelial cell number. Data represent mean±s.e.m. (n=3-13 independent samples) and were analyzed by Student′s t-test. *P<0.01; **P<0.001. (D) Quantification of endothelial cell number from confocal micrographs of 50 hpf wild-type or alk1 MO Tg(fli1a:nEGFP)y7 embryos treated with tricaine between 42 and 50 hpf. The increase in endothelial cell number observed at 40-48 hpf in alk1 morphants is reduced by stopping blood flow. Data represent mean±s.e.m. (n=4-8 independent samples) and were analyzed by Student′s t-test. **P<0.001 |
|
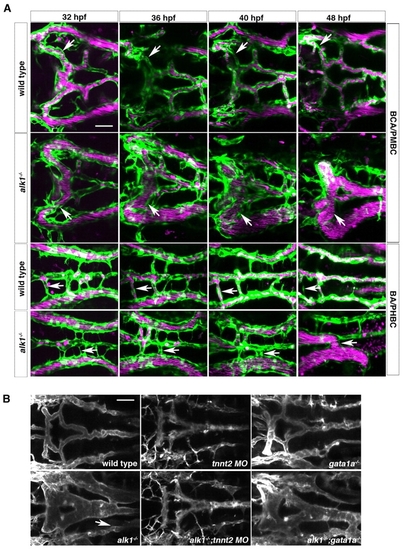
Retention of normally transient arteriovenous connections in alk1 mutants is flow dependent. (A) In wild-type embryos (row 1), transient connections between the basal communicating artery (BCA) and primordial midbrain channel (PMBC) carry blood at 32 hpf but regress by 48 hpf (arrows). In alk1 mutants (row two), one or both of these bilateral connections may be retained, forming a BCA-to-PMBC AVM (arrows). More posteriorly, lumenized connections drain the basilar artery (BA) to the primordial hindbrain channel (PHBC) in wild-type embryos at early times, but almost all regress by 48 hpf (row 3, arrows). In alk1 mutants, one or more of these connections may be retained, forming a BA-to-PHBC AVM (row 4, arrows). Images are two-dimensional confocal projections of Tg(kdrl:GFP)la116; Tg(gata1:dsRed)sd2 embryos, dorsal views, anterior leftwards. Endothelial cells are green; erythrocytes are magenta. (B) AVMs (arrows) are detectable in 48 hpf alk1-/- embryos and in alk1-/-;gata1a-/- embryos (which lack erythrocytes), but not in alk1-/-;tnnt2 MO (which lack blood flow). Images are two-dimensional confocal projections of Tg(fli1a.ep:mRFP-F)pt505 embryos, dorsal views, anterior leftwards. Scale bars: 50 μm. |
|
alk1 expression requires blood flow. (A) Spatiotemporal pattern of alk1 mRNA expression assayed by whole-mount in situ hybridization. Expression is detected in the first aortic arch (AA1, white asterisk), lateral dorsal aorta (LDA; blue arrowhead) and internal carotid artery (ICA, blue arrow) at 26-28 hpf, then in the caudal division of the internal carotid artery (CaDI, white arrow) and basal communicating artery (BCA, white arrowhead) by 28-30 hpf. Tracings in the far right column represent all vessels expressing cdh5 at 36 hpf, with alk1-positive arteries in pink, alk1-negative arteries in red and veins in black or gray. BA, basilar artery; LDA, lateral dorsal aortae; MtA, metencephalic artery; OA, optic artery; PCS, posterior communicating segments. Lateral and dorsal views, anterior leftwards. Frontal view, anterior rightwards. Scale bar: 50 μm. (B) Onset of blood flow correlates with alk1 expression. At 26 hpf, blood flows caudally through AA1 (asterisk) and the LDA (blue arrowhead). The cranialward ICA (blue arrow) and CaDI (white arrow) carry flow by 28 hpf; the BCA (white arrowhead) by 30 hpf; and the PCSs (blue asterisks) by 32 hpf. Images are two-dimensional confocal projections of chrna1-/- (paralyzed), Tg(kdrl:GFP)la116;Tg(gata1:dsRed)sd2 embryos. Endothelial cells are green, erythrocytes are magenta. Lateral and dorsal views (row 2, columns 3-5), anterior leftwards. Frontal view (row 2, columns 1-2), anterior rightwards. Scale bar: 50 μm. (C) Whole-mount in situ hybridization demonstrates that alk1 is downregulated in the absence of blood flow (tnnt2a-/- or tricaine treatment, 32-40 hpf) but is not affected by the absence of erythrocytes (gata1a-/-). cdh5 expression is unchanged under all conditions. All embryos are at 36 hpf except tricaine treated, which are at 40 hpf. Lateral views, anterior leftwards. Scale bar: 100 μm. |
|
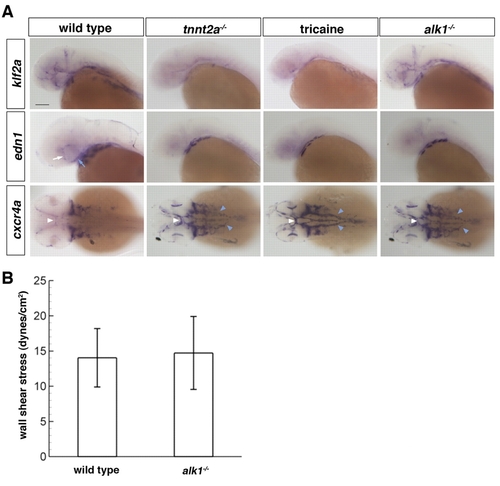
Loss of alk1 dysregulates expression of flow-responsive genes but does not alter shear stress. (A) Whole-mount in situ hybridization using klf2a, edn1 and cxcr4a riboprobes. Vascular klf2a expression is strongly downregulated in the absence of blood flow (tnnt2a-/- or tricaine treatment, 32-40 hpf) but is unaltered in alk1-/- embryos. By contrast, vascular edn1 expression is downregulated and cxcr4a expression is upregulated both in the absence of flow and in alk1 mutants. Changes in expression are primarily in alk1-positive arteries: ICA (blue arrow) and CaDI (white arrow), BCA (white arrowhead) and LDA (blue arrowhead), as well as AA1 (not shown). Lateral expression of both edn1 and cxcr4a is in the pharyngeal arches and is unchanged in all conditions. All embryos are 36 hpf except tricaine treated, which are 40 hpf. klf2a and edn1, lateral views, anterior leftwards. cxcr4a, dorsal view, anterior leftwards. Scale bar: 100 μm. (B) Blood flow in the distal region of AA1 was imaged in chrna1-/- (paralyzed) Tg(kdrl:GFP)la116;Tg(gata1:dsRed)sd2 alk1 mutant embryos and wild-type/heterozygous siblings at 32-36 hpf by high-speed confocal microscopy, and particle image velocimetry used to calculate wall shear stress. Results represent mean±s.d., n=8 wild type and n=5 alk1 mutants. Differences were not significant according to Student′s t-test. EXPRESSION / LABELING:
|
|
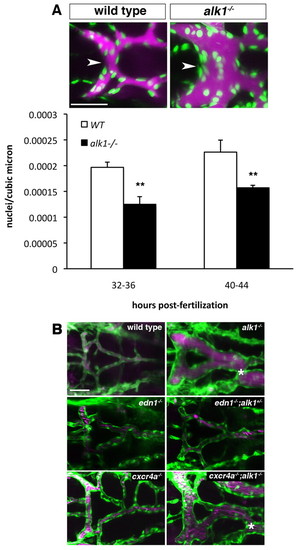
Vasodilation contributes to increased vessel caliber in alk1 mutants. (A) Qtracker 655 non-targeted quantum dots were injected into the common cardinal vein of wild-type and alk1 mutant Tg(fli1a:nEGFP)y7 embryos. Images are two-dimensional confocal projections, dorsal views, anterior leftwards. Embryos aged 32-36 hpf are shown. Endothelial cell nuclei are green; quantum dots are magenta. Scale bar: 50 μm. Endothelial cell density in the BCA (white arrowhead) was calculated from these micrographs by dividing the number of nuclei by the vessel volume. Data represent mean±s.e.m. (n=5-7 independent samples) and were analyzed using Student′s t-test, **P<0.001. (B) Analysis of the contribution of edn1 loss and cxcr4a upregulation to AVM development. AVM development in alk1-/- embryos (asterisk) is not phenocopied by edn1-/- embryos even in the presence of heterozygous levels of alk1 (edn1-/-;alk+/-). cxcr4a-/- embryos do not exhibit AVMs, whereas cxcr4a-/-;alk1+/- embryos invariably develop AVMs, demonstrating that cxcr4a upregulation is not necessary for AVM development in alk1 mutants. Two-dimensional confocal projections of Tg(fli1a:EGFP)y1;Tg(gata1:dsRed)sd2 or Tg(kdrl:GFP)la116;Tg(gata1:dsRed)sd2 embryos. Endothelial cells are green; erythrocytes are magenta. Dorsal views, anterior leftwards. Scale bar: 50 μm. |
|
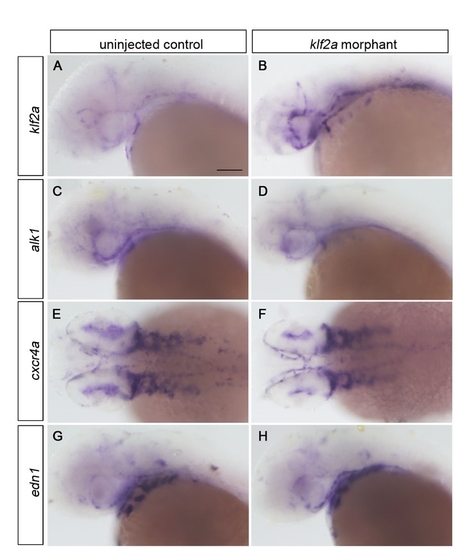
Expression analysis of flow responsive genes in klf2a morphants. (A-H) Embryos were injected with 10 ng klf2a morpholino at the one- to four-cell stage and expression was analyzed at 36 hpf via whole-mount in situ hybridization. Although klf2a expression was consistently upregulated in klf2a morphants, alk1, cxcr4a and edn1 expression was unaffected. (A-D,G-H) Lateral view, anterior leftwards. (E,F) Dorsal views, anterior leftwards. Scale bar: 100 μm. EXPRESSION / LABELING:
|
|
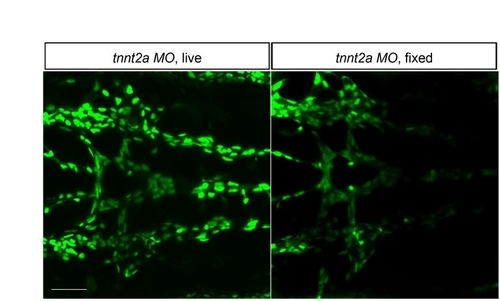
Endothelial cell counts in fixed embryos are comparable with live embryos. Endothelial cells in the BCA/PCS were counted in five live Tg(fli1a:nEGFP)y7 tnnt2a morphants at 48 hpf, then counted again after fixation. Results revealed no difference in cell counts, with an average (mean±s.e.m.) of 41.2±1.35 cells in live embryos versus 40±1.09 cells in fixed embryos. It is difficult to count cells in collapsed vessels, so these images represent the greatest counting challenge. Two-dimensional confocal z-projections, dorsal views, anterior leftwards. Scale bar: 50 μm. |
|
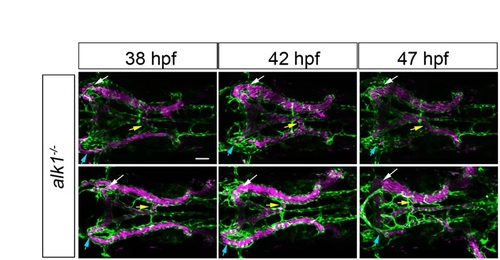
Inter- and intra-embryo variability in AV shunt formation in alk1 mutants. All alk1 mutants initially drain the basal communicating artery via bilateral connections to the primoridal midbrain channel (white and blue arrows), and drain the basilar artery via a variable number of connections to the primordial hindbrain channel (yellow arrows). These connections are also present in wild-type embryos but regress as this arterial system develops (see Fig. 1, main text). By contrast, at least one of these connections is maintained in alk1 mutants. The complement of shunts varies from embryo to embryo, and the prominence of individual shunts in a single embryo changes over time. Each row represents a single alk1-/-; chrna1-/-;Tg(kdrl:GFP)la116;Tg(gata1:dsRed)sd2 embryo imaged at indicated time points. Endothelial cells are green, erythrocytes are magenta. Two-dimensional confocal z-projections, dorsal views, anterior leftwards. Scale bar: 50 μm. |
|
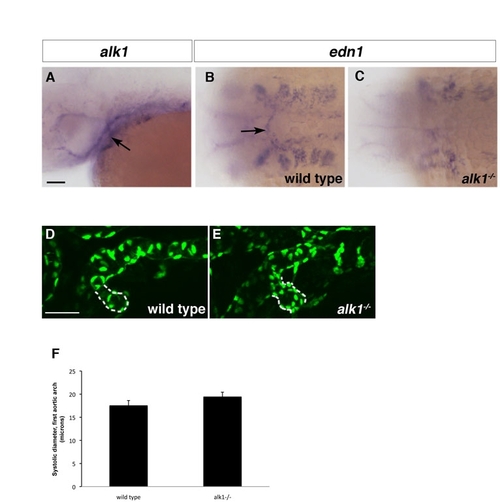
The first aortic arch is alk1 positive and abnormal in alk1 mutants. (A-C) In situ hybridization showing alk1 (A) and edn1 (B,C) expression in 36 hpf wild-type (A,B) and alk1-/- embryos. alk1 is expressed in the first aortic arch at 36 hpf (A, arrow). edn1 is expressed in the first aortic arch in wild-type embryos (B, arrow) but expression is lost in alk1 mutants (C). (D,E) Two-dimensional projections of confocal z-series 36 hpf Tg(fli1a:nEGFP)y7 embryos. The first aortic arch is enlarged in alk1 mutants (E) compared with wild-type siblings (D). However, at this early time, differences are not yet statistically significant (F). Data in F represent an average of three systolic diameters per embryo measured from high speed confocal micrographs, mean±s.e.m. n=8 wild type; n=5 alk1-/-. (A,D,E) Lateral views, anterior leftwards. (A,D) left side. (E) right side. (B,C) dorsal view, anterior leftwards. Scale bars: 50 μm. EXPRESSION / LABELING:
|