- Title
-
FoxA transcription factors are essential for the development of dorsal axial structures
- Authors
- Dal-Pra, S., Thisse, C., and Thisse, B.
- Source
- Full text @ Dev. Biol.
|
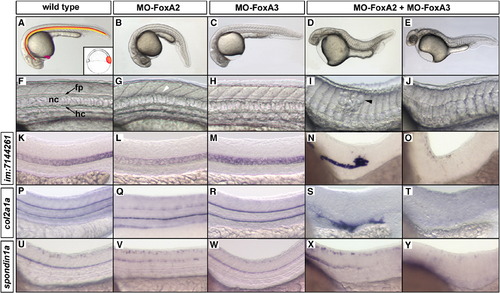
FoxA2 and FoxA3 have redundant activities essential for the formation of axial structures. (A) Schematic representation of the axial structures in a wild-type embryo at 24 hpf (hatching gland, pink; floor plate, red; notochord, orange; hypochord, yellow) and of their progenitors (inset) at the dorsal margin of early gastrula. (B) FoxA2 morphant is ventrally curved and shows failure in floor plate differentiation (white arrowhead in G). (C) FoxA3 morphant lacks the hatching gland but develops the rest of axial structures. (D–E) FoxA2–FoxA3 double morphants are strongly impaired for notochord development. Mild phenotype (D) is characterized by a caudal truncation and by local disorganizations (black arrowhead in I) of the notochord. (E) Strong phenotype is characterized by a complete lack of notochord. (F–J) High magnification of the truncal region of embryos presented in A–E, that shows floor plate (fp), notochord (nc) and hypochord (hc). (K–Y) Analysis of molecular markers specific of notochord (im:7144261, K–O), floor plate and hypochord (col2a1a, P–T) and floor plate (spondin1a, U–Y) at 24 hpf in the trunk region. Embryos are in lateral views, anterior to the left. |
|
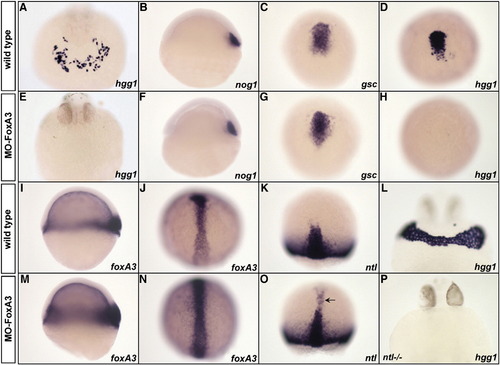
Phenotype of FoxA3 morphants. (A and E) FoxA3 morphants do not present any cells expressing the hatching gland marker hgg1 at 24 hpf (E) compared to wild-type (A). (B–D, F–H). Analysis of prechordal plate (nog1 and gsc) and hatching gland (hgg1) marker expression during gastrulation in wild-type (B–D) and FoxA3 morphants (F–H). At shield stage, nog1 expression is normal in FoxA3 morphant (F). At 80% epiboly, gsc is correctly expressed in FoxA3 morphant (G), while hgg1 expression is absent (H). (I–J, M–N) foxA3 expression is increased at early (M) and late gastrula stage (N) in FoxA3 morphants compared to wild-type (I and J). (K and O) ntl is ectopically expressed in the prechordal plate of FoxA3 morphant (arrowhead, O) compared to wild-type (K) at 80% epiboly. (L and P) Injection of MO-FoxA3 prevents the formation of hatching gland in ntl/ mutant at 30 hpf. (A, E, L, and P) Frontal views, dorsal up. (B, F, I, and M) Lateral views, animal pole up. (C, D, G, H, J, K, N, and O) Dorsal views, animal pole up. EXPRESSION / LABELING:
PHENOTYPE:
|
|
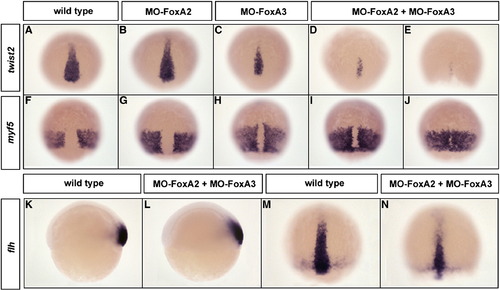
FoxA2 and FoxA3 are required for the maintenance but not for the induction of axial mesoderm. (A–J) Expression at 80% epiboly of the chordamesoderm marker twist2 (A–E) and the paraxial mesoderm marker myf5 (F–J) in FoxA2 (B and G) or FoxA3 (C and H) and FoxA2–FoxA3 (D, E, I, and J) morphants. twist2 and myf5 expressions are identical to wild-type (A and F) in FoxA2 morphants or slightly affected in FoxA3 morphants. In double FoxA2–FoxA3 morphants, axial mesoderm is narrower (D) or almost absent (E), while paraxial mesoderm is dorsally extended (I) or partially fused along the dorsal midline (J). (K–N) flh expression domain in FoxA2–FoxA3 morphants is not affected at the shield stage (L) and is narrower at 80% epiboly (N) compared to wild-type (K and M). Dorsal views, anterior up except (K and L) lateral views. EXPRESSION / LABELING:
PHENOTYPE:
|
|
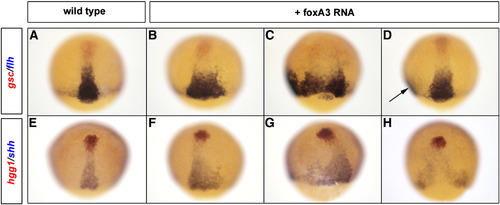
Gain of FoxA2 or FoxA3 function enlarges the axial mesoderm territory. Analysis of the axial mesoderm territory at midgastrula stage with two combinations of prechordal plate (red)/notochord (blue) markers, gsc/flh (A–D) and hgg1/shh (E–H). Embryos injected with foxA3 RNA present an enlargement of flh (B–D) and shh (F–H) expression domains compared to wild-type (A and E). When the axial mesoderm is massively enlarged, it splits in two distinct axes (arrow in D). Dorsal views, anterior up. EXPRESSION / LABELING:
|
|
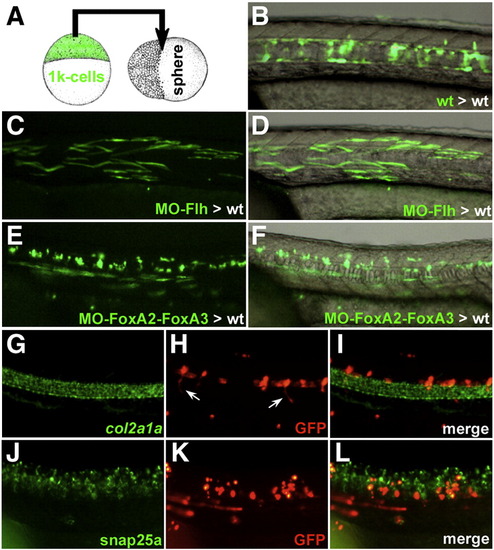
Heterochronic transplantation of FoxA2–FoxA3 morphant cells in wild-type embryos. (A) Schematic representation of the heterochronic transplantation strategy. (B) Wild-type cells labeled with GFP, heterochronically transplanted in the marginal region of a wild-type host embryo populate the axial structures (notochord, hypochord and floor plate) at 24 hpf. (C and D) Transplanted Flh morphant cells can form muscle fibers at 24 hpf. (E and F) Donor cells inactivated for FoxA2–FoxA3 can form muscle fibers and spinal cord neurons. (G–L) Molecular identity of the transplanted FoxA2–FoxA3 morphant cells assessed by double labeling of GFP (H and K) and the axial structures marker col2a1a (G) or the spinal cord neurons marker snap25a (J). The GFP positive cells never express col2a1a (I) but are present in the ventral neural tube where they differentiate into neurons (including motor neurons extending their axons, arrows in (H) and are located next to snap25a expressing cells (L). (B–L) Lateral views of the trunk region, anterior to the left. |
|
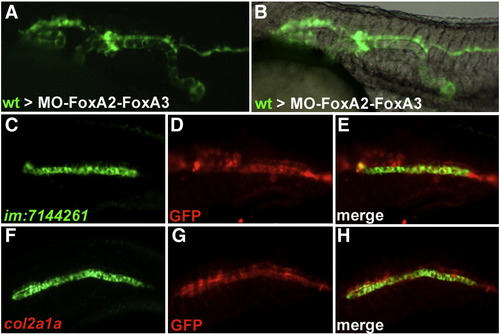
Wild-type cells transplanted into FoxA2–FoxA3 morphant embryos form axial structures in a cell autonomous manner. (A and B) Trunk region of FoxA2–FoxA3 morphants at 24 hpf after transplantation of wild-type cells labeled with GFP. Wild-type cells form stretches of axial structures composed exclusively of transplanted cells. (C–H) Identity of the transplanted cells analyzed by labeling of GFP (D and G) with the notochord marker im:7144261 (C) or the floor plate and hypochord marker col2a1a (F). All cells expressing col2a1a and im:7144261 are also labeled for GFP (E and H). Lateral views, anterior to the left. |
|
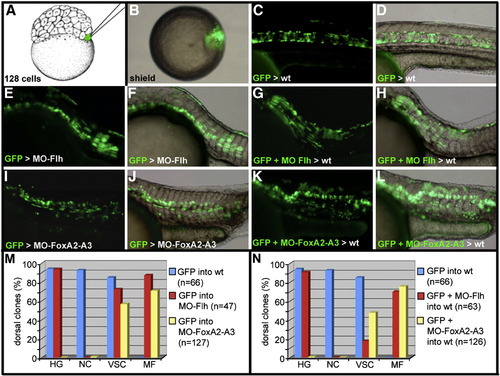
Cell-lineage analysis of dorsal midline progenitors. (A–D) Schematic representation of the cell-lineage strategy. (A) One marginal blastomere of a 128-cell stage embryo is injected with GAP-gfp RNA. (B) At early gastrula stage, embryos displaying the GFP positive cells within the embryonic shield are isolated. The fate of the labeled cells is analyzed at 24 hpf. (C and D) In wild-type embryos, GFP positive cells populate only the axial structures and the dorsal periderm. (E–H) Dorsal marginal cells labeled with GFP and depleted of Flh activity differentiate into muscle fibers and floor plate in Flh morphant embryos (E and F) and in wild-type embryo (G and H). (I–L) Dorsal marginal cells labeled with GFP and depleted of FoxA2 and FoxA3 activities participate in the formation of muscle fibers and cells of the ventral neural tube in FoxA2–FoxA3 morphants (I and J) and in wild-type embryos (K and L). (M and N) Quantitative analysis of the repartition of the GFP-labeled cells in the hatching gland (HG), notochord (NC), ventral spinal cord (VSC) and in muscle fibers (MF), when they differentiate in a morphant (M) or wild-type environment (N). (A) Lateral view, anterior up. (B) Animal pole view, dorsal to the right. (C–L) Lateral views, anterior to the left. |
|
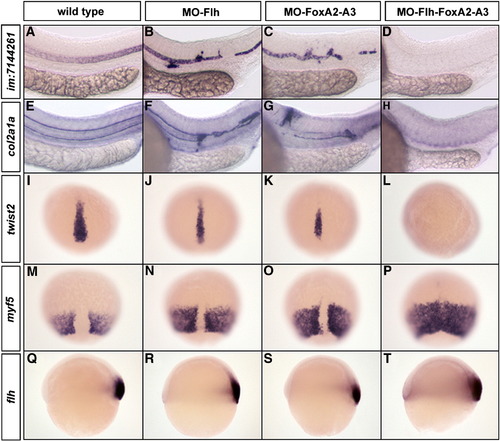
Flh and FoxAs transcription factors are both essential for the maintenance of axial structures. (A–H) Expression at 24 hpf of the notochord marker im:7144261 (A–D) and the floor plate and hypochord marker col2a1a (E–H). Embryos injected with low dose of MO-Flh (0.8 ng) (B and F) or MO-FoxA2–FoxA3 (3 x 4 ng) (C and G) display weak phenotypes compared to wild-type (A and E). With the same amount of morpholinos, the triple inactivation of Flh, FoxA2 and FoxA3 completely abolishes notochord (D), floor plate and hypochord (H) development. (I–P) Expression at 80% epiboly of the chordamesoderm marker twist2 (I–L) and paraxial mesoderm marker myf5 (M–P). Partial Flh (J and N) or FoxA2–FoxA3 (K and O) morphants show a slight reduction of axial mesoderm. Partial Flh-FoxA2–FoxA3 triple morphants lack all axial mesoderm (L) that is accompanied by a complete fusion of paraxial mesoderm along the dorsal midline (P). (Q–T) Expression of flh at the shield stage in strong Flh (R), FoxA2–FoxA3 (S) and Flh-FoxA2–FoxA3 (T) morphants is similar to wild-type embryo (Q). (A–H) Lateral views, anterior to the left. (I–P) Dorsal views, anterior up. (Q–T) Lateral views, dorsal to the right. |
|
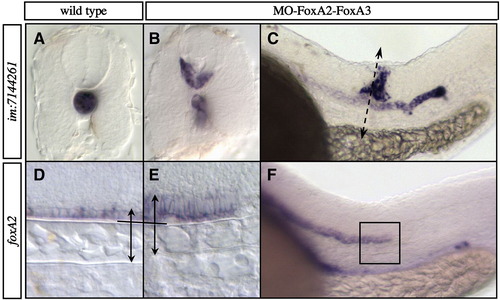
Abnormal development of the axial structures remaining in partial FoxA2–FoxA3 morphants. Expression of notochord (im:7144261) (A–C) and floor plate markers (foxA2) (D–F) at 24 hpf. (A and B) Transverse sections showing that the notochord is smaller in FoxA2–FoxA3 morphants compared to wild-type, and that some of these cells are mislocated in the ventral neural tube. (C) Position of the transverse section shown in B. (D and E) In FoxA2–FoxA3 morphant, floor plate cells are elongated along the dorso-ventral axis of the neural tube, instead of being cuboidal. (F) Position of the area shown in E. (A and B) Transverse sections, dorsal up. (C–F) Lateral views, anterior to the left. |
|
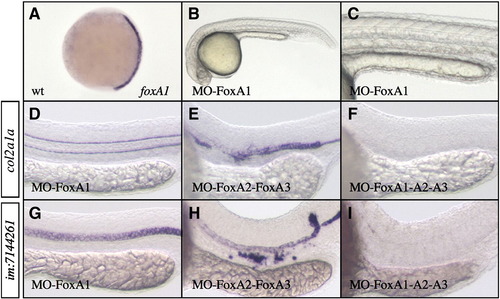
FoxA1 loss of function strongly amplifies FoxA2–FoxA3 phenotype. (A) foxA1 expression is initiated at tail bud stage in axial mesoderm. (B and C) FoxA1 morphant presents normal axial structures at 24 hpf. (D and G) Loss of FoxA1 alone has no effect on axial structures development but (F and I) strongly increases the strength of FoxA2 and FoxA3 shown in (E and H). Triple FoxA1-FoxA2–FoxA3 morphants lack all axial structures. While col2a1a and im:7144261 expressions are normal in FoxA1 morphants (D and G), they are completely absent in triple FoxA1-FoxA2–FoxA3 morphants (F and I; 98% n = 55 for im:7144261). (A) Dorsal views, anterior up. (B–I) Lateral views, anterior to the left. |
|
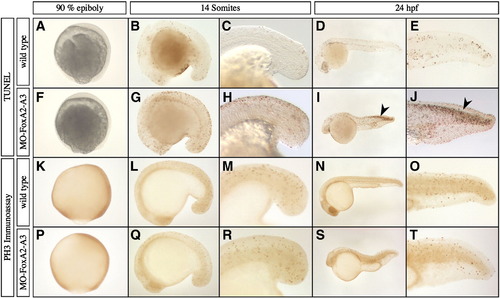
Analysis of cell proliferation and apoptosis in FoxA2 and FoxA3 morphants. (A–J) Analysis of apoptosis by TUNEL assay at 90% epiboly, 14 somites and 24 hpf. The pattern of apoptotic cells in FoxA2–FoxA3 morphants is similar to wild-type embryos at every stage, except in the dorsal neural tube at 24 hpf, that shows strong apoptosis (arrowheads in I and J). (K–T) Analysis of cellular proliferation by phospho-histone H3 immunoassay at 90% epiboly, 14 somites and 24 hpf. FoxA2–FoxA3 morphants are similar to wild-type embryos at all stages. (A–T) Lateral views, anterior to the left (except in A, F, K, and P with animal pole up). (C, E, H, J, M, O, R, and T) Magnification of the tail region. PHENOTYPE:
|
|
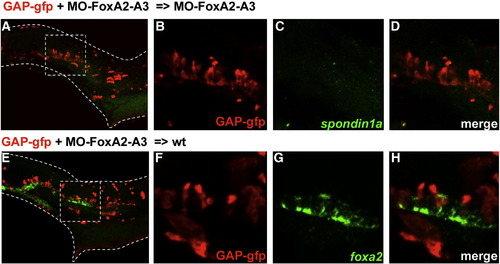
Identity of FoxA2–FoxA3 morphant cells located in the ventral neural tube. Dorsal marginal cells labeled with GFP and depleted of FoxA2 and FoxA3 activities give rise to cells located in the ventral neural tube at 24 hpf in a FoxA2–FoxA3 morphant (A–D) and in a wild-type embryo (E–H). Identity of labeled cells determined by double labeling of GFP (B and F) with the floor plate markers spondin1a (C) or foxA2 (G). GFP-labeled cells never express the floor plate markers (D and H). Squares in A and E indicate the region magnified in B–D and F–H, respectively. Lateral views, anterior to the left. |
|
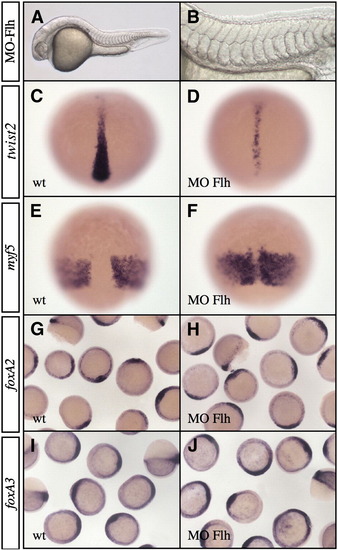
Phenotype of floating head morphant. (A and B) Embryos injected with 1.6 ng MO-Flh do not develop notochord and hypochord and present a patchy floor plate and fused somites at 24 hpf. (B) High magnification of the trunk region of Flh morphants. (C–F) Analysis of twist2 (C and D) and myf5 (E and F) expression at 80% epiboly. In Flh morphants, twist2 expression domain is narrower (D) and myf5 territory is partially fused along the dorsal midline (F). (G–J) At shield stage, expressions of foxA2 (G and H) and foxA3 (I and J) are not affected in Flh morphants (H and J) compared to wild-type (G and I). (A and B) Lateral views, anterior to the left. (C–F) Dorsal views, anterior up. (G–J) Animal pole views. EXPRESSION / LABELING:
|
|
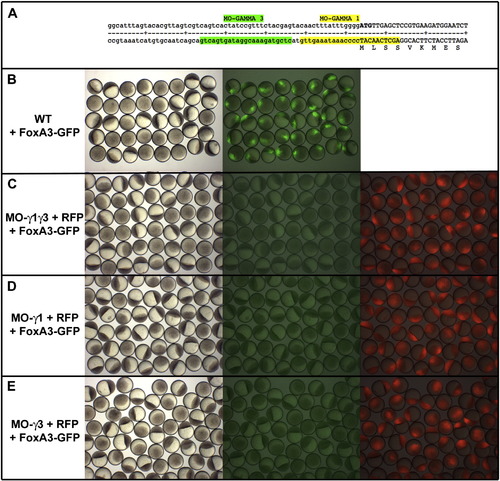
FoxA3 morpholinos efficiently block translation of FoxA3-GFP fusion protein. (A) Sequence of the 5′UTR and of the region coding for the Nt part of the FoxA3 protein showing the position of FoxA3 morpholinos, MO-γ1 (that covers the initiation of translation) and MO- γ3 (two nucleotides upstream of MO- γ1 in the 5′UTR part of the RNA). (B) Injection of 50 pg of RNA coding for a FoxA3-GFP fusion protein at the 4 cell stage results in embryo displaying a strong fluorescence in the nucleus of one quarter of the cells. (C) Injection of 8 ng of both MO- γ1 and MO- γ3 as well as (D) 8 ng of MO- γ1 or (E) 8 ng of MO- γ3 abolish the translation of FoxA3-GFP fusion protein (central panels in C–E compared to right panel in A). Morpholinos have been injected with a RNA coding for a RFP used as control to show that all embryos observed are morphants (right panels in C–E). |
|
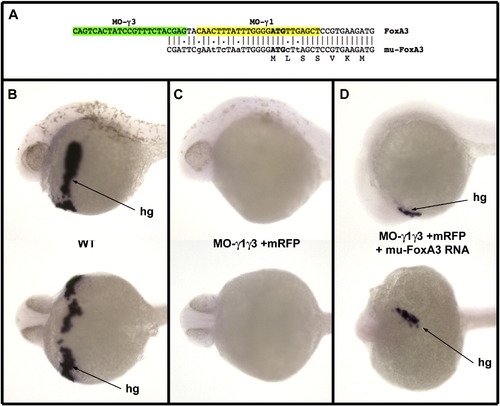
Rescue of FoxA3 morphants by a FoxA3 RNA mutated at the level of the morpholino target sequences. (A) Sequence of the 5′ region of the mutated FoxA3 construct. This mutant overlaps only with three of the four last nucleotides, which hybridizes to MO-g3, and six mismatches have been introduced in the sequence, which hybridizes to MO-γ1. (B) In situ hybridization with Hgg1 a specific marker for the hatching gland (hg) at 30 hpf in lateral view (top) and ventral view (bottom) visualizing the differentiated hatching gland in wild-type embryo. (C) Injection of 8 ng of each MO-γ1 and MO-γ3 results in embryos devoid of all hgg1 expressing cells (58/58 injected embryos). (D) Injection of 8 ng of each morpholino at the one cell stage followed by injection of 25 pg of the mutated FoxA3 RNA in a marginal blastomere at the 32 cell stage results in embryos displaying Hgg1 expressing cells in 11 out of 46 injected embryos (injection in marginal blastomeres at late stage generate clones expressing FoxA3 which overlap at least partially with the prechordal plate territory in about 25% of the embryos). |
Reprinted from Developmental Biology, 350(2), Dal-Pra, S., Thisse, C., and Thisse, B., FoxA transcription factors are essential for the development of dorsal axial structures, 484-495, Copyright (2011) with permission from Elsevier. Full text @ Dev. Biol.